ARENES
1. Write a structural formula:
a) allylbenzene e) 2,4,6-tribromoaniline
b) m-nitrotoluene f) m-xylene
c) p-bromophenol g) (Z)-1-phenyl-1-butene
d) o-ethylanisole h) m-chlorostyrene
2. Give an IUPAC names:
a) b) c) d) e)
![]()
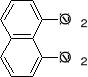
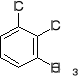
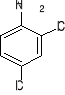
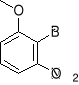
3. Write structural formulas and give acceptable names for all the isomeric
a) nitrotoluenes c) tetrafluorobenzenes
b) dichlorobenzoic acids d) tribromophenols
4. Give the structure of the expected product from the reaction of isopropylbenzene (cumene) with:
3 mol H2 / Pt
b) Na/ NH3, EtOH (Birch reduction)
Na2Cr2O7, H2SO4, H2O
NBS / CCl4
5. Give the structure of the expected product from the reaction of ethylobenzene with:
a) Br2, h b) Br2 / AlBr3
6. Each of the following may be represented at least one alternative resonance structure. Write
such a resonance
a) b) c) d)

![]()
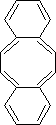
![]()
7. Give reagents suitable for effecting each of the following reaction, and write the principal
products:
nitration of tert-butylbenzene
nitration of phenol
bromination of toluene
sulfonation of anisole
Friedel-Crafts acylation of benzene with benzoyl chloride
8. Write equations showing how you could prepare each of the following from benzene or
toluene:
sec-butylbenzene h)1-bromo-2,4-dinitrobenzene
1-bromo-2-phenylpropane i) 3-bromo-5-nitrobenzoic acid
4-tert-butyl-benzyl bromide j) 2-bromo-4-nitro-benzoic acid
1-chloro-3-nitrobenzene k) diphenylmethane
p-isopropylbenzenesulfonic acid l) 1-phenyl-1-octene
3-bromo-4-methylacetophenone m) 1-phenyl-1-ctyne
2-bromo-4-ethyltoluene n) 1,4-di-tert-butyl-1,4-cyclohexadiene
9. Write equations showing how you could prepare each of the following from anisole and any necessary organic or inorganic reagents:
p-methoxybenzenesulfonic acid c) p-methoxystyrene
2-bromo-4-nitroanisole d) 4-bromo-2-nitroanisole
10. Suggest reagents suitable for carrying out each of the following conversions. In most cases more than one synthetic operation may be necessary.
Ph-Et → Ph-CHBr-CH3
Ph-CHBr-CH3 → Ph-CHBr-CH2Br
Styrene → phenylacetylene
Phenylacetylene → 1-phenylbutane
Ph-CH2CH2OH → 4-phenyl-1-butyne
11. Suggest a suitable series of reactions for carrying out each of the following synthetic
transformations:
a) b) c)
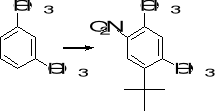
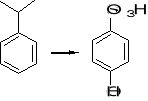
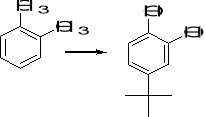
12. What combination of acyl chloride or acid anhydride and arene would you choose to
prepare each of the following compounds by a Friedel-Crafts acylation reaction?
a) b) c) d)
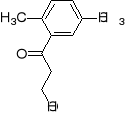
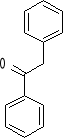
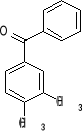
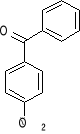
1
Wyszukiwarka
Podobne podstrony:
Chemia organiczna czesc I poprawiona
chemia organiczna wykład 6
Wykład 9 CHEMIA ORGANICZNA
Chemia Organiczna 4
Chemia organiczna IV
CHEMIA- CHEMIA ORGANICZNA, CHEMIA
bromoacetanilid, Studia, Sprawozdania, Chemia organiczna
Przykladowy egzamin chemia organiczna - ICiP - 2010-zima. , Egzamin
I POPRAWKA EGZAMINU Z CHEMII ORGANICZNEJ, Technologia chemiczna, Chemia organiczna, 4 semestr, organ
chemia organiczna 2003 cała PDF(1)
więcej podobnych podstron