
International Biodeterioration & Biodegradation 52 (2003) 97–100
www.elsevier.com/locate/ibiod
Production of xylan-degrading enzymes from Amazon forest fungal
species
Roseli Garcia Medeiros
a
, Rog-erio Hanada
b
, Edivaldo Ximenes Ferreira Filho
a;∗
a
Departamento de Biologia Celular, Laboratorio de Enzimologia, Universidade de Brasilia, Brasilia, DF, CEP 70910–900, Brazil
b
Laboratorio de Patologia da Madeira, Instituto Nacional de Pesquisa da Amazˆonia, Manaus, Amazˆonia, Brazil
Received 1 June 2002; received in revised form 25 October 2002; accepted 19 November 2002
Abstract
Ten fungal species were isolated from decomposing wood in the Amazon forest. All produced xylan-degrading enzymes when cultivated
in liquid media containing oat spelt xylan. The best producing strains were identi9ed as Penicillium corylophilum, Aspergillus niger and
Trichoderma longibrachiatum. The best yields of -xylosidase and -arabinofuranosidase activities were Trichoderma harzianum and
Trichoderma sp. Xylanase activities from crude extract samples of P. corylophilum, A. niger and T. longibrachiatum were partially
characterized. They were most active at 40
◦
C (A. niger) or 45
◦
C (P. corylophilum and T. longibrachiatum) and pH 4.0–4.5. Reducing
agents (-mercaptoethanol and dithiothreitol),
L
-cysteine and
L
-tryptophan activated xylanase activity. In addition, dithiothreitol improved
the half-lives of these enzymes at 50
◦
C and 60
◦
C. By contrast, N-bromosuccinimide inhibited all the enzyme activities. Xylan and
dithiothreitol a>orded protection against xylan-degrading enzyme inactivation by N-bromosuccinimide, but failed to reverse it. The apparent
K
m
values on soluble and insoluble xylans from oat spelt showed that xylan-degrading enzymes from A. niger, T. longibrachiatum and
P. corylophilum were most active on the soluble form.
? 2003 Elsevier Ltd. All rights reserved.
Keywords: Xylanolytic fungi; Xylan; Xylan-degrading enzymes
1. Introduction
Xylans are branched polysaccharides found in the cell
walls of all land plants and composed of a backbone
containing -1, 4-linked-
D
-xylosyl residues and, depend-
ing on their source and extraction procedure, with side
chains of acetyl, glucuronic, methylglucuronic, arabino-
furanosyl, ferulic and coumaric groups (
). Xylans account for 20–35% of the total
dry weight of hardwoods and annual plants and repre-
sent a vast resource that can be used for production of
fermentable sugars and fuels (
). The complete cleavage of xylan is carried out
by the synergistic action of -xylanase and its accessory
enzymes, including -xylosidase, -arabinofuranosidase,
-methylglucuronidase and acetyl xylan esterase (
). Xylan-degrading enzymes have been reported
∗
Corresponding author. Fax: +55-612-734608.
E-mail address:
(E.X.F. Filho).
to be produced by a wide variety of fungal species (
). These enzymes have potential application in
sacchari9cation of biomass and biobleaching of kraft pulps
). The objectives of the
present work were to isolate and identify fungal species from
the Amazon forest and to evaluate their capacity to produce
xylan-degrading enzyme activity during growth in liquid
medium containing oat spelt xylan as the carbon source.
We also characterized the crude xylan-degrading enzyme
activities from Penicillium corylophilum, Aspergillus niger
and Trichoderma longibrachiatum.
2. Materials and methods
2.1. Chemicals
Oat
spelt
xylan,
carboxymethyl
cellulose,
p-nitrophenyl--
D
-xyloside,
p-nitrophenyl--
L
-arabino-
furanoside,
L
-cysteine, -mercaptoethanol, dithiothreitol
0964-8305/03/$ - see front matter ? 2003 Elsevier Ltd. All rights reserved.
doi:10.1016/S0964-8305(02)00179-8

98
R. Garcia Medeiros et al. / International Biodeterioration & Biodegradation 52 (2003) 97–100
Table 1
Identity and source of isolates used for production of xylan-degrading enzymes
Fungus (species)
Isolate number
Source (wood)
Paecilomyces variotti Bainier
LPM 48
Virola sp.
Trichoderma longibrachiatum Rifai
LPM 139
Scleronema micranthum Ducke
Trichoderma harzianum Rifai
LPM 296
Couroupita guianensis Aubl.
Penicillium roquefortti Thom
LPM 303
Hura creaptans L.
Penicillium sp.
LPM 494
Simaruba amara Aubl.
Aspergillus niger van Tieghen
LPM 93
Cedrelinga cateniformis Ducke
Penicillium corylophilum Dierckx
LPM 119
Parkia multijuga Benth
Fusarium sp.
LPM 495
Simaruba amara Aubl.
Trichoderma sp. strain SM
LPM 496
Sche?era morototoni Decne and Planch
Trichoderma sp. strain SA
LPM 497
Simaruba amara Aubl.
(DTT), N-bromosuccinimide (NBS) and
L
-tryptophan were
purchased from Sigma Chemical Co. (St. Louis, MO).
2.2. Organism and enzyme production
All fungi species were isolated from decomposed wood
in the natural forest reserve of INPA (National Research
Institute of Amazonia, Brazil). The origins of isolates of
10 species of wood-inhabiting fungi are shown in Table
Pieces (10–20 mm
2
) of decomposed wood were placed in
a series of Petri plates containing 3% malt-agar and 250 mg
chloramphenicol, and incubated at 25
◦
C for 1 month. After
incubation, each puri9ed isolate was transferred to fresh 3%
malt-agar medium. The cultures may incubated at 25
◦
C until
the mycelium spread over most of the medium surface. They
were then stored at 5
◦
C. The identi9cation of fungi species
was done according to the protocols and have been previ-
ously described, the species and genus features described
in the following references:
. The puri9ed fungi were transferred to plates that con-
tained MYG (0.2% malt extract, 0.2% yeast extract, 2%
glucose and 2% agar) or BDA (2.0% potato broth, 2% glu-
cose and 1.5% agar) media. T. longibrachiatum and Tri-
choderma harzianum were maintained in MYG medium,
while Paecilomyces variotti, Trichoderma sp. strain SM,
Trichoderma sp. strain SA, Penicillium roquefortti, Peni-
cillium sp., P. corylophilum, Fusarium sp. and A. niger
were maintained in BDA medium at 28
◦
C. For produc-
tion of xylanase activity, the fungi were cultured in Er-
lenmeyer Lasks containing 0:5% (w=v) oat spelt xylan in
100 ml of minimal medium (0.7% KH
2
PO
4
, 0.2% K
2
HPO
4
,
0.05% MgSO
4
7H
2
O, 0.1% (NH
4
)
2
SO
4
) supplemented with
0.06% yeast extract. Flasks were inoculated with 2:9 ×
10
3
=ml of spore suspensions from routine subcultures. Cul-
tures were grown at pH 7.0 for 7 days at 28
◦
C with shak-
ing at 100 rpm. Subsequently the content of each Lask was
9ltered through Whatman 9lter paper number 1. The su-
pernatant solutions, hereafter called crude extracts, obtained
from 9ltration procedure were stored at 4
◦
C for subsequent
use as xylan-degrading enzyme preparations.
2.3. Enzyme assays
Xylan-degrading enzyme activity was determined, ex-
cept where noted otherwise, by mixing 50 l of enzyme
solution with 100 l of oat spelt xylan in 100 mM sodium
acetate bu>er, pH 5.0 at 50
◦
C for 30 min. The release
of reducing sugar was measured using the dinitrosalicylic
reagent method (
). Xylan-degrading
enzyme activity was expressed as mol reducing sugar
formed min=ml enzyme solution, i.e., as IU/ml. The activity
of the enzyme preparations was also performed in the pres-
ence of amino acid modifying (-mercaptoethanol, NBS,
and DTT), and aminoacids (
L
-tryptophan and
L
-cysteine).
The reaction mixtures contained individual reagents at a
9nal concentration of 0.1 or 4:6 mM (NBS) and 9:3 mM
(-mercaptoethanol, DTT,
L
-tryptophan and
L
-cysteine).
Appropriate controls were included in all cases. Xylan,
DTT and
L
-tryptophan were also pre-incubated for 10 min
with crude enzyme sample just before addition of NBS
and the assay was carried out as above. The crude enzyme
sample was also pre-incubated with NBS for 10 min prior
addition of DTT. Carboxymethyl-cellulase assay was per-
formed in the conditions as described above. The activity
against 9lter paper was measured as described by
. -xylosidase and -arabinofuranosidase ac-
tivities were determined as reported elsewhere (
). For the kinetic experiments, soluble and
insoluble samples from oat spelt xylan were used as substra-
tes in concentration ranges of 0.15–0:9 mg=ml (soluble xy-
lan) and 0.15–2:0 mg=ml (insoluble xylan). The substrates
were prepared as described by
. K
m
val-
ues were estimated from Michaelis–Menten equation with a
non-linear regression data analysis program (
Leatherbarrow,
1987
). The determination of optimum temperature of
xylan-degrading enzyme samples was carried out in the tem-
perature range of 30–80
◦
C at pH 7.0. Optimum pH values
were determined by measuring the enzyme activity at 50
◦
C
at pH values from 3.0 to 8.0. The following bu>ers were
used: 50 mM sodium acetate bu>er (3.0–5.5), and sodium
phosphate bu>er (6.0–8.0). The bu>ers, regardless of pH,
were adjusted to the same ionic strength with NaCl. The
R. Garcia Medeiros et al. / International Biodeterioration & Biodegradation 52 (2003) 97–100
99
temperature stability of xylan-degrading enzyme samples
was determined by pre-incubating the enzyme at 50
◦
C and
60
◦
C. At various times, aliquots were withdrawn and the
residual activity was measured under standard conditions.
2.4. Protein concentration
Protein concentration was measured by the method of
, using bovine serum albumin as standard.
3. Results and discussion
3.1. Screening of xylan-degrading enzyme activities
All fungal species were able to produce reducing sugars
from xylan (results not shown). Most of the crude extract
samples showed weak but measurable -xylosidase and
-arabinofuranosidase activities. The enzymes were most
active in T. harzianum and Trichoderma sp strain SM.
Fusarium sp. did not show xylosidase activity. The best
producers of xylan-degrading enzyme activity were P. cory-
lophilum, A. niger and T. longibrachiatum, respectively.
Most of the enzyme samples did not contain cellulase
activity (9lter paper-degrading activity or carboxymethyl
cellulase). Only A. niger, Trichoderma sp. strain SM and
P. corylophilum were able to degrade, at a low level, 9lter
paper and carboxymethyl cellulose (results not shown).
3.2. Characterization of xylan-degrading enzyme activity
from crude extracts of P. corylophilum, A. niger and
T. longibrachiatum
Xylan-degrading enzyme activities from crude extract
samples of P. corylophilum, A. niger and T. longibrachia-
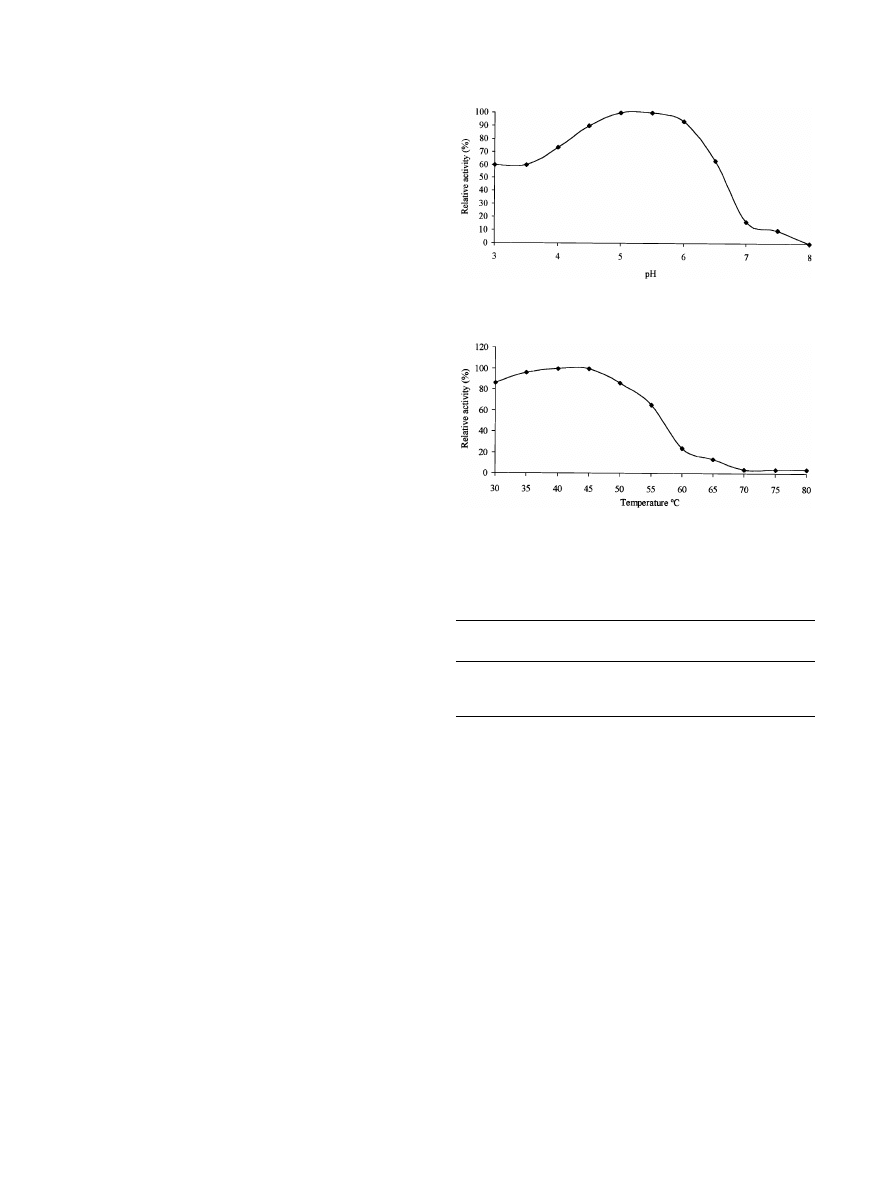
tum were partially characterized. The pH and temperature
were typical of xylan-degrading enzymes from mesophilic
fungi (
). Those from P. corylophilum
and T. longibrachiatum were most active at 45
◦
C, while A.
niger had a maximum enzyme activity at 40
◦
C. The enzyme
samples displayed highest activity at pH range of 4.5–5.5.
The e>ect of pH and temperature on xylan-degrading en-
zyme activity from P. corylophilum is presented in Figs.
and
, respectively. All enzyme samples showed more aNn-
ity against soluble xylan (Table
). The xylan-degrading
enzyme activity from A. niger showed the lowest K
m
value.
The involvement of some modifying agents on xylan-
degrading enzyme activities from crude extract samples
of P. corylophilum, A. niger and T. longibrachiatum
was investigated. Crude enzyme samples from T. longi-
brachiatum and A. niger were activated by
L
-cysteine,
L
-tryptophan, DTT and -mercaptoethanol (Table
DTT and -mercaptoethand did not improve the xylan-
degrading enzyme activity from P. corylophilum. The crude
xylan-degrading enzymes were inhibited by NBS at 4:6 mM.
At 0:1 mM NBS, the xylan-degrading enzyme activities of
P. corylophilum, A. niger and T. longibrachiatum were not
Fig. 1. E>ect of pH on xylan-degrading enzyme activity from P.
coryophilum.
Fig. 2. E>ect of temperature on xylan-degrading enzyme activity from P.
coryophilum.
Table 2
K
m
Determination of xylan-degrading enzyme activities from fungal
species on soluble and insoluble xylans
Fungus
Soluble xylan
Insoluble xylan
(mg/ml)
(mg/ml)
Trichoderma longibrachiatum
0.7
1.2
Penicillium corylophilum
0.6
0.8
Aspergillus niger
0.5
1.0
a>ected (results not shown).
L
-Tryptophan is believed to
be involved in the substrate-binding of the xylan-degrading
enzymes (
). One must be cautious in deter-
mining the mode of inhibition since NBS is a strong oxi-
dizing agent and is capable of eliciting a variety of e>ects
on proteins (
). Evidence for involvement of the
L
-tryptophan residue at the active site is given by the com-
plete protection of the crude xylan-degrading enzyme ac-
tivity from P. corylophilum by 1% xylan and DTT against
NBS. On the other hand, xylan and DTT failed to reverse
the xylan-degrading enzyme of P. corylophilum from in-
activation by NBS (results not shown).
The industrial application of xylan-degrading enzymes
in bio-bleaching process for paper pulp requires ther-
mostable enzymes (
). The e>ect of temperature
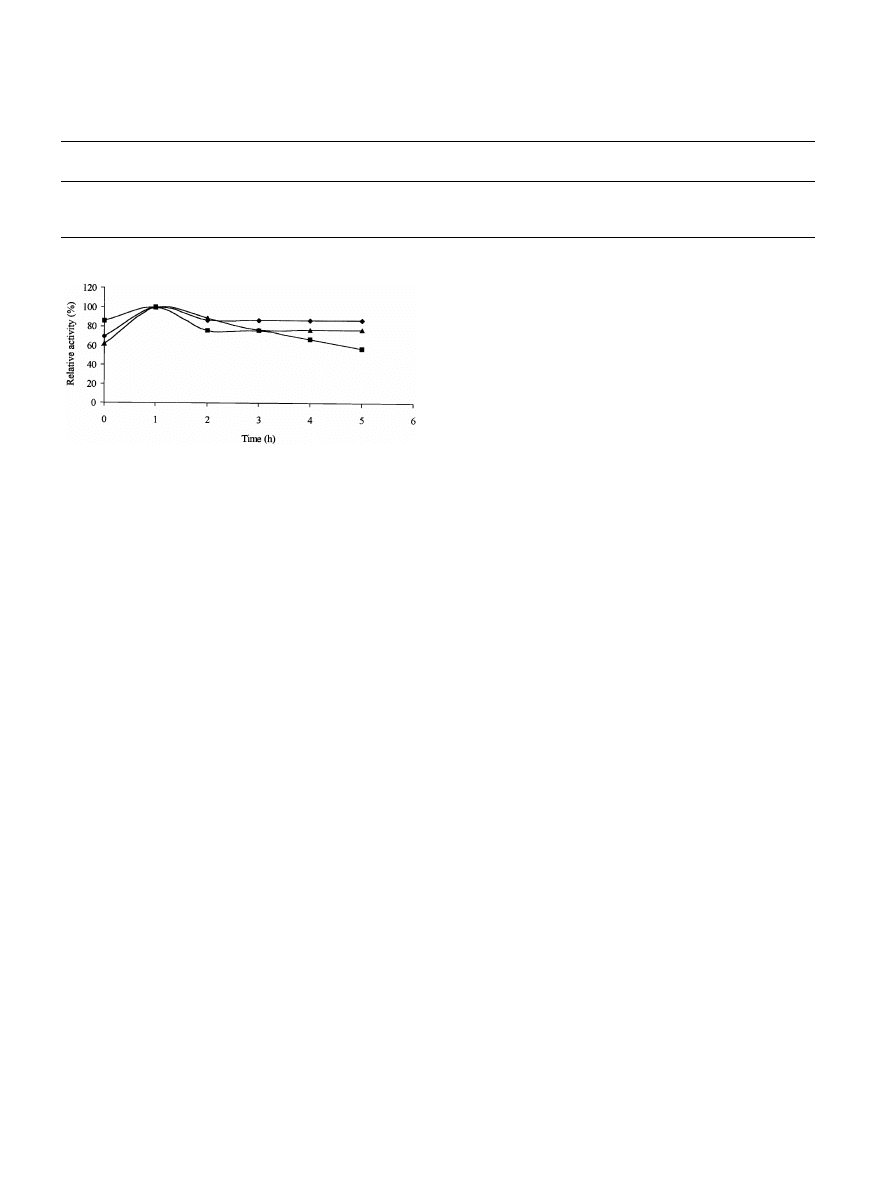
on the stability of the xylan-degrading enzyme activities
was therefore determined. After 6 h incubation at 45
◦
C
and pH 5.5, all enzyme samples lost 50% of their initial
activity. Xylan-degrading enzymes from P. corylophilum,
T. longibrachiatum and A. niger retained 40%, 47% and
100
R. Garcia Medeiros et al. / International Biodeterioration & Biodegradation 52 (2003) 97–100
Table 3
E>ect of reducing agents,
L
-tryptophan and
L
-cysteine on xylan-degrading enzyme activities from fungal species
Fungus
L
-Cysteine
-Mercaptoetanol
DTT
L
-Tryptophan
(% control)s
(% control)
(% control)
(% control)
Trichoderma longibrachiatum
160
380
180
260
Penicillium corylophilum
141
100
100
125
Aspergillus niger
145
145
109
181
Fig. 3. Thermal stability of xylan-degrading enzymes from P. cory-
lophilum (), T. longibrachiatum (
4
) and A. niger ().
65% of their activity, respectively, after incubation at 50
◦
C,
pH 5.5 for 60 min. The presence of DTT in the incu-
bation mixture markedly increased the thermostability of
xylan-degrading enzyme samples. Xylan-degrading enzyme
activity from A. niger was the most thermostable of the
three enzyme samples, with a half-life of greater than 5 h
at 60
◦
C and pH 5.5 while those from P. corylophilum and
T. longibrachiatum showed half-lives of 1 h. At 50
◦
C and
pH 5.5, all enzyme samples showed half-lives greater than
6 h (Fig.
This present research work was done to select fungal
strains for application in kraft pulp pre-bleaching process.
P. corylophilum produced the highest xylan-degrading en-
zyme activity, followed by A. niger and T. longibrachiatum
when grown in medium containing oat spelt xylan as the car-
bon source. To our knowledge, this is the 9rst report on the
production and characterization of a crude xylan-degrading
enzyme sample from P. corylophilum. Further studies will
be carried out to purify and determine the role of the puri-
9ed enzyme systems on the hydrolysis of xylan, their use in
biobleaching and the involvement of
L
-tryptophan, cysteine
and other amino acid residues in enzymatic activity.
Acknowledgements
This work was supported by research grants from Bio-
amazˆonia/BASA (Brazil) and PADCT III/CNPq (Brazil).
EX.F.F. and R.G.M. are recipient of research fellowship and
postgraduate maintenance scholarship, respectively, from
CNPq (Brazil).
References
Booth, C., 1971. The Genus Fusarium. Commonwealth Mycological
Institute, Kew, England, 1–237.
Bradford, M.M., 1976. A rapid and sensitive method for the quantization
of microgram quantities of protein utilizing the principle of protein
dye binding. Analytical Biochemistry 72, 248–254.
Carmichael, J.W., Brycekedrick, W., Conners, L.L., Sigler, L. 1980.
General of Hyphomycetes. The University of Alberta Press, Canada,
pp. 1–386.
Clarke, A.J., 1987. Essential tryptophan in cellulase from Schizophyllum
commune. Biochimica et Biophysica Acta 912, 424–431.
Coughlan, M.P., 1992. Towards an understanding of the mechanism of
action of main chain-hydrolyzing xylanases. In: Visser, J., Beldman, G.,
Someren, M.A.K.-van, Voragen, A.G.J. (Eds.), Xylans and Xylanases.
Elsevier Science, Amsterdam, pp. 111–139.
Filho, E.X.F., 1998. Hemicellulase and biotechnology. In: Pandalai,
S.G. (Ed.), Recent Research Development in Microbiology. Research
Signpost, Trivandrum, -India, pp. 165–176.
Filho, E.X.F., Puls, J., Coughlan, M.P., 1993. Biochemical characteristics
of two endo--1,4-xylanases produced by Penicillium capsulatum.
Journal of Industrial Microbiology 11, 171–180.
Haltrich, D., Nidetzky, B., Kulbe, K.D., Steiner, W., Zupancic, S., 1996.
Production of fungal xylanases. Bioresource Technology 58, 137–161.
Klich, M.A., Pitt, J.I. 1988. A laboratory guide to the common Aspergillus
species and their teleomorphs. Commonwealth Scienti9c and Industrial
Research Organization, Australia, pp. 1–116.
Kulkarni, N., Shendye, A., Rao, M., 1999. Molecular and biotechnological
aspects of xylanases. FEMS Microbiology Reviews 23, 411–456.
Leatherbarrow, R.J. 1987. Enz9tter: a non-linear regression data analysis
program for the IBM PC. Biosoft, London, pp. 1–91.
Lee, Y-E., Lowe, S.E., Zeikus, G., 1993. Regulation and characterization
of xylanolytic enzymes of Thermoanaerobacter saccharolyticum
B6A-RI. Applied and Environmental Microbiology 59 (3), 763–771.
Mandels, M., Andreotti, R., Roche, C., 1976. Measurement of
saccharifying cellulase. Biotechnology Bioengineering Symposium 16,
21–33.
Pitt, J.I., 1991. A laboratory guide to common Penicillium species.
Commonwealth Scienti9c and Industrial Organization, Division of
Food Processing, pp. 1–187.
Rifai, M.A., 1969. A revision of the genus Trichoderma. Mycological
papers, Vol. 116. pp. 1–56.
Samson, R., 1974. Paecilomyces and some hyphomycetes. Studies in
Mycology 6, 1–119.
Silveira, F.Q.P., Sousa, M.V., Ricart, C.A.O., Milagres, A.M.F.,
Medeiros, C.L., Filho, E.X.F., 1999. A new xylanase from a
Trichoderma harzianum strain. Journal of Industrial Microbiology and
Biotechnology 23, 682–685.
Smith, D.C., Bhat, K.M., Wood, T.M., 1991. World Journal of
Microbiology and Biotechnology 7, 475–484.
Ximenes, F.A., Silveira, F.Q.P., Filho, E.X.F., 1996. Production of
-xylosidase activity by Trichoderma harzianum strains. Current
Microbiology 33, 71–77.
Document Outline
- Production of xylan-degrading enzymes from Amazon forest fungal species
Wyszukiwarka
Podobne podstrony:
Xylan Degrading enzymes from Aspergillus niger
xylan degrading enzymes from Melanocarpus
Xylan degrading enzymes from the Yeast
Screening for distinct xylan degrading enzymes in complex shake flask
Production of Energy from Biomass Residues 020bm 496 1993
Techniques to extract bioactive compounds from food by products of plant origin
SMeyer WO8901464A3 Controlled Process for the Production of Thermal Energy from Gases and Apparatus
the illict preparation of morphine and heroin from pharmaceutical products containing codeine homeba
A xylan degrading strain of Sulfolobus solfataricus
~$Production Of Speech Part 2
An%20Analysis%20of%20the%20Data%20Obtained%20from%20Ventilat
Blacksmith The Origins Of Metallurgy Distinguishing Stone From Metal(1)
78 1101 1109 Industrial Production of Tool Steels Using Spray Forming Technology
Mankiewicz Boczek, J i inni Bacteria homologus to Aeromonas capable of microcystin degradation (201
GB1008594A process for the production of amines tryptophan tryptamine
pair production of black holes on cosmic strings
BoyerTiCS Religious Thought as a By Product of Brain Function
Production Of Speech Part 1
więcej podobnych podstron