
EFFECT OF VARIOUS DRYING METHODS ON TEXTURE AND
COLOR OF TOMATO HALVES
GHOLAM REZA ASKARI, ZAHRA EMAM-DJOMEH
1
and
MARYAM TAHMASBI
Transfer Phenomena Laboratory, Department of Food Science, Technology and
Engineering, Faculty of Biosystems Engineering, Agricultural Campus
University of Tehran
Karaj, 31587-11167, Iran
Accepted for Publication May 6, 2009
ABSTRACT
Tomatoes were pretreated with osmotic solutions (NaCl and sucrose) at
different concentrations and then dried using hot air (75C, 1.5 m/s), a vacuum
(55C, 75 kPa) or hot-air drying followed by microwave treatment (400 W,
10 s). The effects of pretreatment and drying method on the drying kinetics
were examined. A puncture test and scanning electron microscopy were used
to analyze the effects of these processes on texture and microstructure. Hunter
values (L, a, b) were used to measure color. Measurements showed that two
osmotic solutions, S
3
(40% sucrose, 5% NaCl) and S
4
(40% sucrose, 10%
NaCl), performed better, reducing drying times and having a positive effect on
microstructure, but an adverse effect on hardness. Apart from the type of
process, dehydration reduced firmness and collapsed the structure of tomato
halves. The subsequent microwave treatment then caused further damage,
especially on the surface of the dried samples, but enhanced their color when
combined with appropriate osmotic treatment.
PRACTICAL APPLICATIONS
This study shows that the color and structural changes of tomato during
drying can be reduced using appropriate procedure. This may find application
in the production of dried tomato with better appearance and lower drying cost.
KEYWORDS
Color, drying kinetics, hot-air drying, microstructure, microwave drying,
osmotic pretreatment, texture, vacuum drying
1
Corresponding author. TEL:
+98-21-8879-6165; FAX: +98-21-8879-6165; EMAIL: emamj@ut.ac.ir
Journal of Texture Studies 40 (2009) 371–389.
© 2009, Wiley Periodicals, Inc.
371

INTRODUCTION
Dehydration is an important process in the chemical and food processing
industries. The basic objective of drying food products is the removal of water
from a solid to the point where microbial spoilage and deteriorating chemical
reactions are greatly minimized. Tomato, being a popular fruit, finds numerous
uses in both fresh and processed forms. Processed products include ketchup,
sauces, pastas and juice. However, drying is not a popular way to process
tomatoes because of its adverse effect on the quality of the final product. The
fruit tissue darkens upon drying (Gupta and Nath 1984) and a strong distinc-
tive flavor develops. Nevertheless, interest in the production of dried tomatoes
is increasing as a result of their potential use in pizza toppings, snacks and
other savory dishes.
A number of methods are used to dry fruits and vegetables. Hot-air drying
is the most common method. However, this method can cause an unpleasant
taste and color and reduce the nutritional content of the product (Silveira et al.
1996; Goula et al. 2006; Toor and Savage 2006). It can also bring about a
decline in porosity and water absorbance capacity and a shifting of the solutes
from the internal part of the drying material to the surface over the long drying
period at high temperatures (Feng and Tang 1998; Drouzas et al. 1999;
Maskan 2001). Also, low thermal conductivity of food materials in the falling
rate period limits heat transfer to the inner part of food during conventional
heating (Feng and Tang 1998).
The elimination of these problems, preventing significant quality loss and
achieving fast and effective thermal processing, has resulted in the increasing
use of microwaves for drying food. Microwave drying is rapid, more uniform
and energy efficient compared with conventional hot-air drying (Drouzas and
Schubert 1996). In the microwave process, energy is converted into the kinetic
energy of the water molecules and then into heat when the water molecules
realign in the changing electrical field and interact with the surrounding
molecules (Mudgett 1989; Khraisheh et al. 1997).
Predrying treatment and drying substantially affect the quality of the
products. Osmotic pretreatment preceding air drying was found to be advan-
tageous to the quality of the products (Collins et al. 1997; Shi et al. 1999;
Lewicki et al. 2002). The combination of osmotic dehydration and microwave-
convective drying has been proposed by a number of researchers for fruits and
vegetables to reduce drying time and introduce into the products solutes such
as sucrose, salt and calcium (Torreggiani 1993; Ertekin and Cakaloz 1996).
In addition, osmotic dehydration is effective at relatively low temperatures
with minimal damage to color and texture (Silveira et al. 1996; Moreno et al.
2000; Valencia Rodriguez et al. 2003; Stojanovic and Silva 2007). However,
there is little information about the effect of combined methods such as
372
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI

osmotic hot-air and osmotic-convective microwave drying on texture, color
and, especially, on the microstructure of dried tomatoes.
The objective of this investigation was to compare the drying kinetics,
texture, color and microstructure of tomato halves using a combination of
drying techniques. The effect of the type of osmotic solution used (brine or
sugar; single or binary) was also evaluated.
MATERIALS AND METHODS
Sample Preparation
Fresh tomatoes (Lycopersicon esculentum var. Roma) obtained from a
local market in Tehran, Iran and were sorted visually for color (bright red),
firmness, size (diameter 4–5 cm) and lack of blemishes. In comparison with
other varieties, Roma has a firm and pulpy tissue with lower moisture content
and is therefore suitable for drying. The fresh tomatoes were placed at an
ambient temperature (20C) for 24 h before the experiments. Prior to drying,
the tomatoes were cut into halves and placed in small hermetic containers.
Three replications were run for each experiment.
Osmotic Pretreatment
The halves were osmotically dehydrated in NaCl and NaCl–sucrose solu-
tions (Table 1) at a regulated temperature (30
⫾ 2C) and agitation of 150 rpm.
The halves (30 g) were placed in 600-mL beakers containing the osmotic
solution and maintained inside a temperature-agitation controlled bath. The
weight ratio of the fruit medium to osmotic medium was less than 1:10 to
avoid significant dilution of the medium and a subsequent decrease of the
driving force during the process. The samples were removed from the solution
at 15, 30, 60, 120, 180 and 240 min of immersion, drained and the excess
TABLE 1.
TYPE OF OSMOTIC SOLUTION
Solution
% NaCl
% Sucrose
S1
5
30
S2
10
30
S3
5
40
S4
10
40
S5
15
30
S6
15
0
373
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES

solution on their surfaces was removed with absorbent paper. Pretreated
samples were completely dried using one of the following drying methods.
Hot-Air Drying
Tomato halves were dried in a pilot plant hot-air drier (tray dryer, Arm-
field, Hampshire, England). The drying was operated at an air velocity of
1.5 m/s parallel to the drying surface of the slices at 75C dry bulb temperature.
The operation mode was controlled using a computer connected to the dryer.
To obtain the drying curves, moisture loss was recorded with a digital balance
(Cobos, Homburg, Germany) at 5-min intervals beginning 30 min after the
start of drying until 30 min before end of drying, after which point it was
measured every 10 min. Hot-air drying was conducted until a moisture content
of 0.2 kg/kg dry matter was reached.
Vacuum Drying
Vacuum conditions were maintained using a vacuum pump and moni-
tored with a manometer. Two steel plates heated by electric resistance provided
the thermal energy. An automatic regulator controlled the temperature of the
plates. The experimental procedure consisted of putting food samples on the
hot plate, closing the door of the chamber and putting the chamber under a
vacuum. Tomato samples were withdrawn from the dryer at set intervals and
their weights determined using an analytical balance with accuracy to 0.001 g.
The temperature of the plate was set at 55C and the pressure of the chamber at
75 kPa.
Microwave-Assisted Hot-Air Drying
Hot-air drying was conducted as previously described until a moisture
content of 0.3 kg/kg dry matter was reached. Initial observation revealed that
using a higher moisture content produced a lower quality product; thus,
samples with a low moisture content of 0.3 kg/kg were used. After this point,
to obtain uniform moisture distribution in the samples, they were placed in a
hermetically sealed container for 30 min. Next, the samples were transferred to
a programmable domestic microwave oven (Butane MR-1, Butane, Tehran,
Iran, maximum output of 1,000 W at 2,450 MHz.) for the microwave treat-
ment. It was observed that charring and sample boiling occurred at 800 and
600 W, respectively. Thus, only the 400 W power level was chosen for 5-, 10-
and 15-s treatment times. Samples were placed at the centre of the turntable in
the microwave (400 W, 10 s). The use of the turntable was necessary to achieve
uniform heating of the samples and to reduce the level of microwave power on
374
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI

the magnetron (Khraisheh et al. 1997). After the microwave treatment, none of
the samples had the same moisture content. The maximum recorded moisture
content was 0.18 kg/kg.
The microwave power available to the load was measured using an IEC
Standard Method 60705 (IEC, 2004) with some modifications. Cold ethanol
was used instead of water to verify the microwave heating efficiency. This
liquid was chosen because of its low dielectric constant (
e
″) and high loss
factor (
e
′) that represent good absorption and low reflection of microwave
energy. The measured efficiency of the cavity was approximately 70% (Pereira
et al. 2007).
Microstructure
Scanning electron microscopy (SEM) was used to analyze microstruc-
tural changes during drying. To obtain the SEM images, small pieces were
taken from both the inner parts and surface of the tomato slices. The samples
were coated with a very thin layer of gold under high vacuum and analyzed
using a scanning electron microscope (XL-30, Philips, Amsterdam, the
Netherlands).
Mechanical Properties
Firmness was evaluated by measuring the stress at maximum force using
a texture analyzer (H5KS-Hounsfield, Redhill, England). Samples were kept at
20C until analysis to minimize the influence of temperature on the textural
results. Stress at maximum force is related to the hardness and firmness of the
samples. Measurements were performed at a constant speed of 1 mm/s using
a cylindrical puncture flat-head probe (d
= 1.6 mm).The samples were cut into
halves using a sharp knife and their texture firmness was analyzed by punching
the newly cut surface of each half. Stress (
s), in MPa, was then calculated
using Eq. (1):
σ
= ×
F
A
10
6
(1)
where F is the maximum force in Newtons read by the texture analyzer and A
is the area of the puncture probe in mm
2
. Changes in textural hardness were
reported as the ratio between the maximum force obtained for treated samples
to that observed for fresh ones (Heredia et al. 2007).
Color
Color evaluation of the tomato samples was performed using a Hunter-
Lab ColorFlex, A60-1010-615 model colorimeter (Hunter-Lab, Reston, VA)
375
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES

that measures lightness (L), redness (a) and yellowness (b) together with their
ratio (a/b), which represents color quality. The desired tomato color properties
are higher L, higher a and lower a/b. The total color difference was calculated
using the following equation, where subscript 0 refers to the color reading of
the fresh apple. Fresh apple was used as a reference and a larger
DE denotes
greater color change from the reference material.
ΔE
L
L
a
a
b
b
=
−
(
)
+
−
(
)
+
−
(
)
0
2
0
2
0
2
(2)
Hue angle (h*) is expressed in degrees: 0° (red), 90° (yellow), 180°
(green) and 270° (blue), and was calculated as
h
b
a
*
=
⎛
⎝
⎞
⎠
−
tan
1
(3)
Water Content
Water content was measured using a vacuum drier at 70C until a constant
weight was achieved (AOAC, 1980; 22.013).
Statistical Analyses
Experiments were conducted in triplicate and an analysis of variance of
the results was carried out using MSTATC software (Michigan State Univer-
sity, East Lansing, MI). The means obtained from each set were compared
using the Duncan’s multiple range test based on a completely randomized
design (0.05 confidence level).
RESULTS AND DISCUSSION
The influence of dehydration time and type of osmotic solution are shown
in Fig. 1. The osmotic process was evaluated in terms of water loss and solid
gain. A typical high rate of water removal (and solid uptake) in the initial
stages was observed, followed by slower removal (and uptake) in later steps. A
number of researchers have reported similar results (Lazarides et al. 1995;
Kowalska and Lenart 2001; Park et al. 2002; Azoubel and Murr 2004).
The effect of the addition of sucrose to water loss and solid gain in binary
systems was investigated. These parameters were higher when salt was used
alone. The lower molecular weight and its effect on reducing water activity
allow it to penetrate fruit tissue at a higher rate. However, as saltiness is
generally undesirable in dried fruits, its use is limited.
376
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI
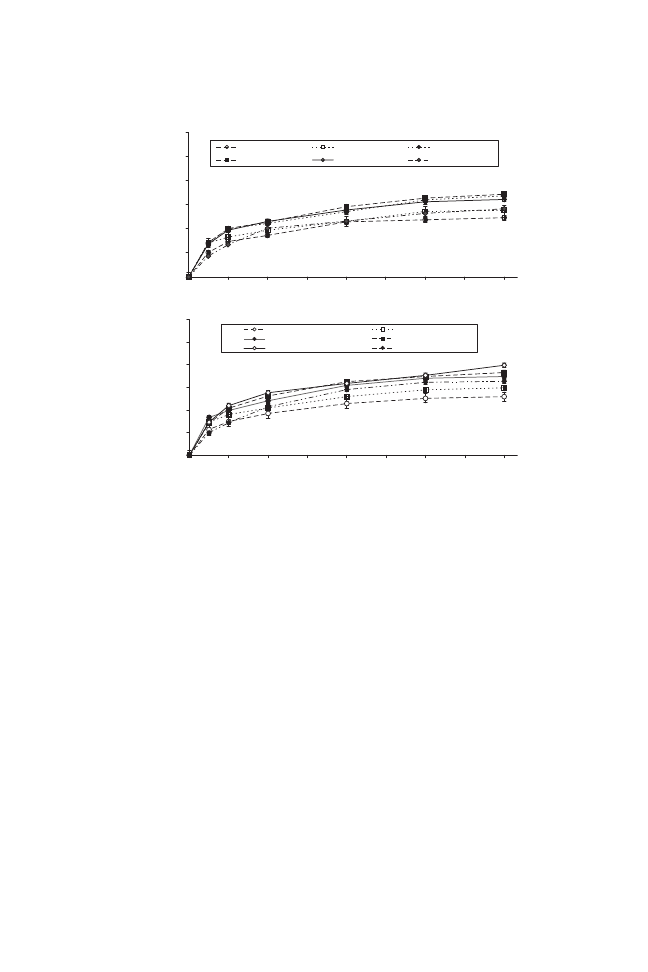
Sucrose can form a barrier layer that reduces water loss and solute
uptake. Two osmotic mediums (S
3
: 40% sucrose
+ 5% NaCl and S
4
: 40%
sucrose
+ 10% NaCl) that showed higher performance were selected for
complementary analysis on the samples. Changes in water loss during 60 min
of dehydration were about 60%, whereas further dewatering, which occurred
between 60 and 180 min, was about 35%. After 180 min, there were no evident
changes in water loss, thus that time was selected as the end of the osmotic
dehydration process.
Complementary drying processes were followed using other drying
methods. Their related curves are shown in the Figs. 2–4. Convective drying
of fresh tomato halves at 75C was a long process requiring 330 min. Evapo-
ration of water from the loose and moist structure of the fresh tomato was
easy. There was no constant rate of evaporation during drying period.
Osmotic pretreatment greatly reduced dehydration time, from 330 to
240 min, for both pretreated samples. There was no significance difference
between the two pretreated samples and repeatability of the process was
good (cv
< 10%).
0.0
0.5
1.0
1.5
2.0
2.5
3.0
0
30
60
90
120
150
180
210
240
TIME (min)
SOLID GAIN (%)
5% NaCl+ 30% sucrose
10% NaCl+ 30% sucrose
5% NaCl+ 40% sucrose
10% NaCl+ 40% sucrose
15% NaCl+ 30% sucrose
15% NaCl+ 0% sucrose
0
10
20
30
40
50
60
0
30
60
90
120
150
180
210
240
TIME (min)
WATER LOSS (%)
5% NaCl+ 30% sucrose
10% NaCl+ 30% sucrose
5% NaCl+ 40% sucrose
10% NaCl+ 40% sucrose
15% NaCl+ 30% sucrose
15% NaCl+ 0% sucrose
FIG. 1. WATER LOSS AND SOLID GAIN DURING OSMOTIC DEHYDRATION OF
TOMATO HALVES
377
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES
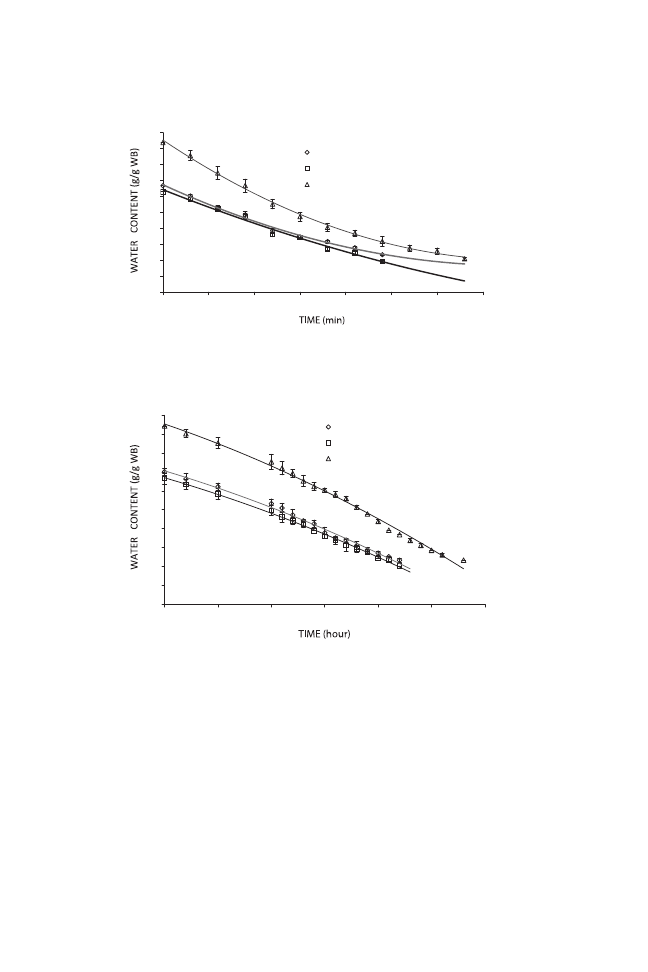
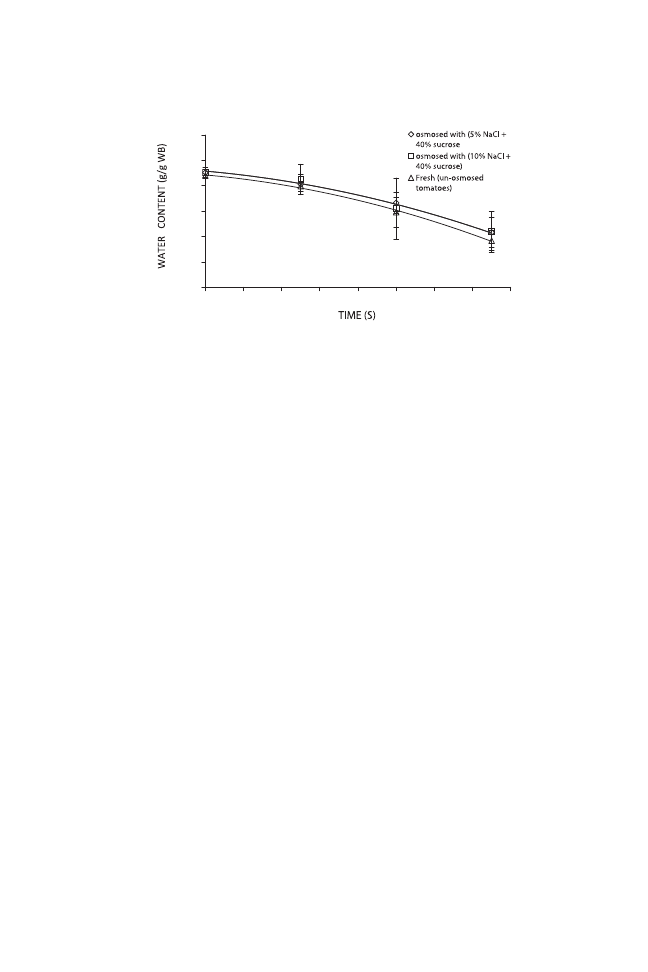
Similar results were obtained for vacuum-dried samples. At low pressure
(75 kPa) and temperature (55C), fresh tomato halves dried in 28 h, whereas
pretreated samples reached the same water content in 22 h (Fig. 3).
The drying kinetics of fresh and pretreated tomato samples during micro-
wave treatment are shown in Fig. 4. At this stage, samples were hot-air dried
until the water content was reduced to 0.3 g/g wet basis for pretreated and
other samples. The semidried samples were then placed in a microwave oven
(P
= 400 W) to finishing the drying process over three time periods (5, 10 and
15 s). As can be seen, treatment for 5 s had no significant effect on water
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
50
100
150
200
250
300
350
osmosed with (5% NaCl + 40% sucrose)
osmosed with (10% NaCl + 40% sucrose)
Fresh (un-osmosed tomatoes)
FIG. 2. HOT-AIR DRYING CURVES FOR FRESH AND PRETREATED TOMATO HALVES
WB, wet basis.
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
5
10
15
20
25
30
osmosed with (5% NaCl + 40% sucrose)
osmosed with (10% NaCl + 40% sucrose)
Fresh (un-osmosed tomatoes)
FIG. 3. VACUUM DRYING CURVES FOR FRESH AND PRETREATED TOMATO HALVES
WB, wet basis.
378
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI
content, although microwave treatment can reduce moisture content signifi-
cantly. The 15 s processing time reduced moisture content, but undesirable
changes such as surface burning and charring occurred. Therefore, 10 s was
chosen as the optimum microwave treatment period for all samples. Micro-
wave treatment reduced drying time to 50 and 75 min for pretreated and
untreated samples, respectively, in only 10 s.
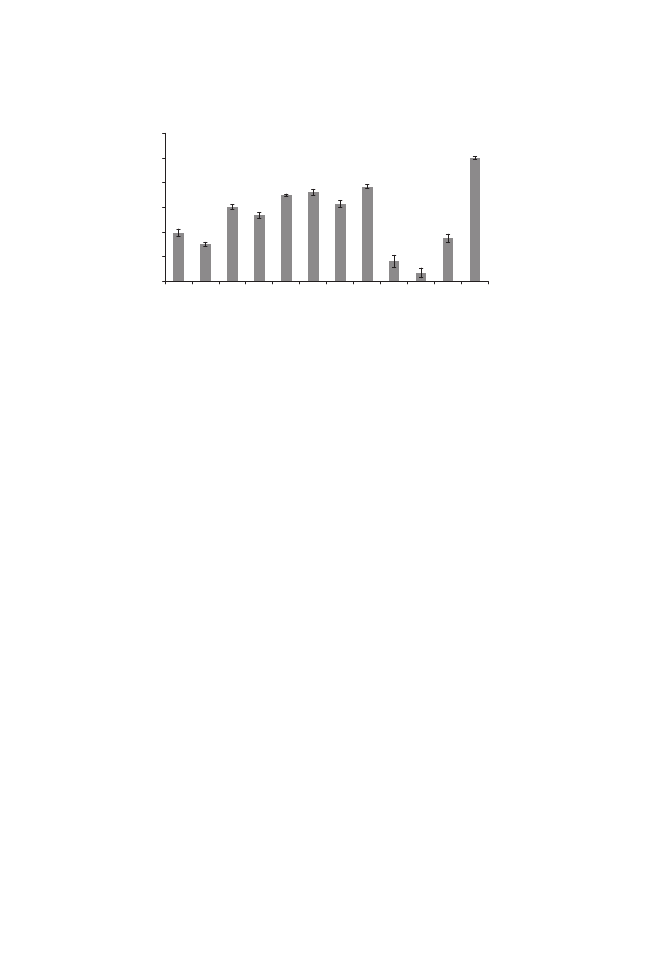
Mechanical changes induced by pretreatment and drying processes were
analyzed in the dried samples. Texture strength change is shown as the ratio
between the maximum forces reached in puncture analysis of the treated
samples to that obtained in puncture analysis of fresh ones. The fresh tomato
samples hypothetically show a value equal to unity (1). Values of less than 1
indicate softening and texture weakening of the samples. The results showed
that osmotic treatment resulted in general softening of the tomato pulp regard-
less of the osmotic solution. Similar results have been reported for tomatoes
(Heredia et al. 2007) and other products (Chirlat and Talens 2005).
In the present study, S
4,
resulting in a higher solid content, softens and
weakens the texture of the samples more than did S
3
. This phenomenon was
observed in the samples after all complementary drying processes. Compa-
rable results for the effects of osmotic solutions were obtained for hot-air,
vacuum and microwave-assisted hot-air drying. In all drying methods, the
pretreated samples had a softer texture at the end of the drying process.
Dehydration made the tomato halves softer than fresh tomatoes. Therefore,
considering the moderate conditions (55C and 75 kPa) in vacuum drying,
higher texture strength was observed in the vacuum-dried samples (Fig. 5).
Greater results were observed for hot-air microwave-dried samples,
which showed less texture strength in the puncture test. Structural collapse and
0.10
0.15
0.20
0.25
0.30
0.35
0.40
0
2
4
6
8
10
12
14
16
FIG. 4. MICROWAVE CONVECTIVE DRYING CURVES FOR FRESH AND PRETREATED
TOMATO HALVES
WB, wet basis.
379
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES
texture weakening during microwave treatment have been reported for other
products (Askari et al. 2006). Increasing salt concentrations had a strong effect
on loss factor (Heredia et al. 2007; De los Reyes et al., 2007) and led to more
texture weakening of microwave-dried tomato halves.
Measured values including total color difference (
DE), color quality (a/b)
and hue angle for all drying methods and pretreatments are presented in
Table 2. It is clear that color parameters are affected apart from any drying
method; however, in this study, there were also significant differences between
the specific drying methods. Comparison shows a noticeable effect between
the osmotic technique and fresh tomatoes for color, but a negligible difference
with the other drying methods. Thus, it can be said that, for dried samples,
there is no significant difference between the osmotic pretreated technique and
other techniques. This indicates that for drying tomatoes, the drying technique
is the main factor affecting color of the dried samples.
The prolonged drying time for the vacuum drying technique caused
higher values of (
DE) than for other methods, but no considerable difference
with the hot-air and microwave-assisted hot-air drying. The a/b values for all
samples showed that microwave-assisted hot-air drying with the application of
an appropriate osmotic treatment is the most suitable technique for drying
tomato halves. The effect of microwave-assisted hot-air drying depended upon
the nature of the osmotic solution. The higher amount of salt in samples treated
with the S
4
osmotic solution led to an increase in the loss factor and in heat
generation, especially in the outer layers of the surface. Therefore, more color
change was observed on the surfaces of these samples. Subsequently, a sig-
nificant decrease in hue angle was observed in the samples treated with
solution 4.
0.0
0.2
0.4
0.6
0.8
1.0
1.2
S3
-os
mo
tic
S4
-os
mo
tic
S3
-ai
r
S4
-ai
r
sh-
air
S3
-va
ccu
m
S4
-va
ccu
m
sh-
vac
cum
S3
-ai
rMW
S4
-ai
rMw
sh-
airM
W
fres
h t
om
ato
F
max .
treated/fresh
FIG. 5. MECHANICAL CHANGE INDUCED BY PRETREATMENT AND DRYING METHOD
IN TOMATO HALVES (OS1, OSMOSED WITH 5% NaCl
+ 40% SUCROSE; OS2, OSMOSED
WITH 10% NaCl
+ 40% SUCROSE)
MW, microwave.
380
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI

Apart from microwave drying, the advantages of osmotic dehydration
were observed in other drying techniques. These effects were also described by
Mujumdar (2000). The effects of osmotic pretreatment on the surface are
shown in Fig. 6. Compared with fresh tomatoes, the surface of the osmotic-
treated samples had a shrunken structure such that there was no evident
cellular structure. There was no apparent difference between treated samples,
but there was some dissimilarity in the stronger osmotic treatment for the S
4
solution as a result of the higher salt concentration. As a consequence, more
shrunken structure is evident in the samples pretreated with the S
4
osmotic
solution.
The effects of hot-air drying on the microstructural properties of the
completely dried samples are shown in Fig. 7. Hot-air drying affected the
surface of the dried samples. Both pretreated and fresh samples were affected,
however, the influence of drying method on the fresh samples was stronger.
The osmotic process preserved samples from undesirable structural changes,
especially for samples treated with the S
4
solution. The surface of treated
samples was softer than for fresh samples that were treated with only hot-air
drying.
As a result of moderate drying conditions in the vacuum drying treatment,
there were no clear differences between the pretreated samples and those dried
only by vacuum drier. However, there was variation in the surfaces only visible
at the microstructural level as shown in Fig. 8. The movement of liquid water
TABLE 2.
EFFECT OF DRYING METHOD AND PRETREATMENT TOTAL COLOR DIFFERENCE (
DE),
COLOR QUALITY (a/b) AND HUE ANGLE OF TOMATO SAMPLES
Drying method
Pretreatment
DE
a/b
Hue angle
Fresh tomato
–
0.00
e
1.64
⫾ 0.05
f
31.37
⫾ 0.78
a
†
S
3
*
0.64
⫾ 0.03
d
1.64
⫾ 0.08
f
31.37
⫾ 0.92
a
S
4
*
0.91
⫾ 0.03
c
2.29
⫾ 0.10
cd
26.27
⫾ 1.24
bc
Hot-air drying
–
1.94
⫾ 0.20
b
3.09
⫾ 0.10
a
17.93
⫾ 0.54
fg
S
3
1.62
⫾ 0.12
b
2.12
⫾ 0.15
cd
25.25
⫾ 1.57
cd
S
4
1.74
⫾ 0.15
b
2.74
⫾ 0.12
b
20.04
⫾ 0.81
ef
Vacuum drying
–
2.92
⫾ 0.20
a
3.34
⫾ 0.15
a
16.66
⫾ 0.76
g
S
3
2.75
⫾ 0.20
a
2.02
⫾ 0.20
de
26.33
⫾ 2.27
bc
S
4
2.72
⫾ 0.25
a
2.39
⫾ 0.19
c
22.70
⫾ 1.63
de
Hot-air microwave drying
–
1.98
⫾ 0.25
b
2.03
⫾ 0.18
de
26.22
⫾ 2.03
bc
S
3
1.73
⫾ 0.25
b
1.80
⫾ 0.24
ef
29.05
⫾ 3.28
ab
S
4
1.83
⫾ 0.30
b
2.15
⫾ 0.29
cd
24.94
⫾ 3.00
cd
* S
3
: 40% sucrose
+ 5% NaCl and S
4
: 40% sucrose
+ 10% NaCl.
† Different superscripts in the same column mean that the values are significantly different at 95%
confidence level (a
= 0.05).
381
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES
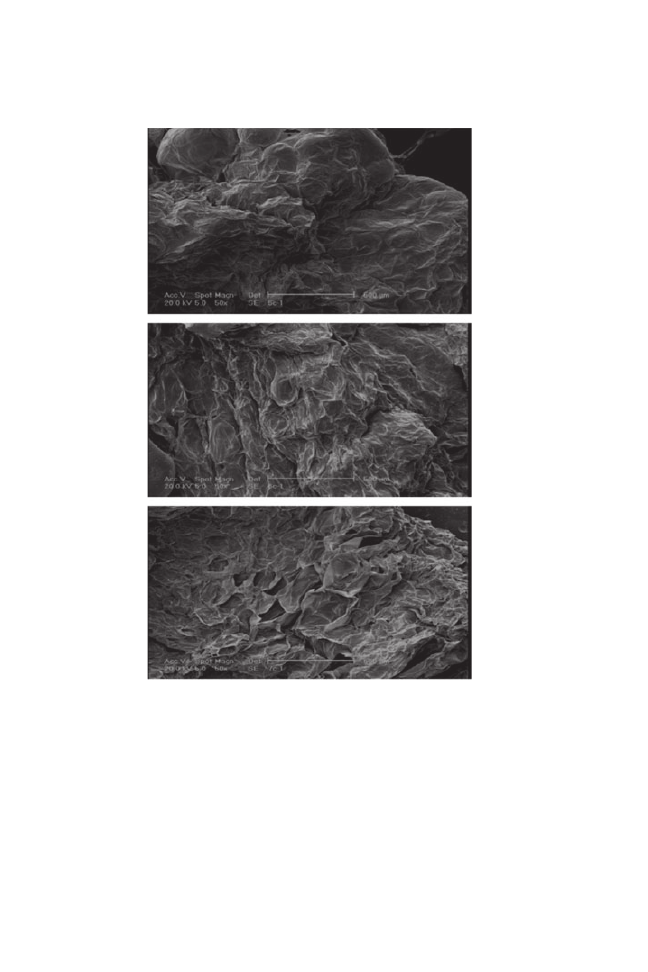
molecules toward the surface under vacuum conditions caused some osmotic
agent on the surface of the treated samples, which apparently led to a smoother
appearance.
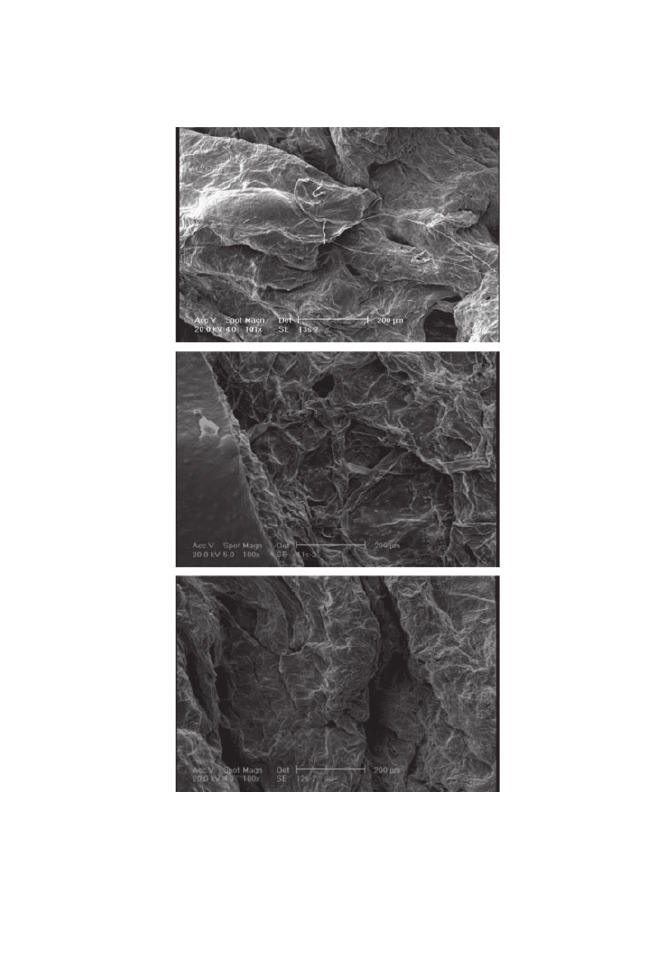
The microstructure of samples affected by the microwave treatment after
hot-air drying is presented in Fig. 9. The rapid conversion of microwave
energy to heat in the internal parts of the samples led to internal pressure that
C
B
A
FIG. 6. SCANNING ELECTRON MICROSCOPY IMAGE OF FRESH (A) AND OSMOTIC
TREATED BY 5% NaCl
+ 40% SUCROSE (B) AND OSMOTIC TREATED BY 10% NaCl + 40%
SUCROSE (C) TOMATO SAMPLES WITH THE SAME MAGNIFICATION (50
¥)
382
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI

FIG. 7. SCANNING ELECTRON MICROSCOPY IMAGE OF FRESH (A) AND OSMOTIC
PRETREATED BY 5% NaCl
+ 40% SUCROSE (B) AND OSMOTIC PRETREATED BY
10% NaCl
+ 40% SUCROSE (C) TOMATO SAMPLES, AFTER HOT-AIR DRYING WITH
THE SAME MAGNIFICATION (100
¥)
383
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES
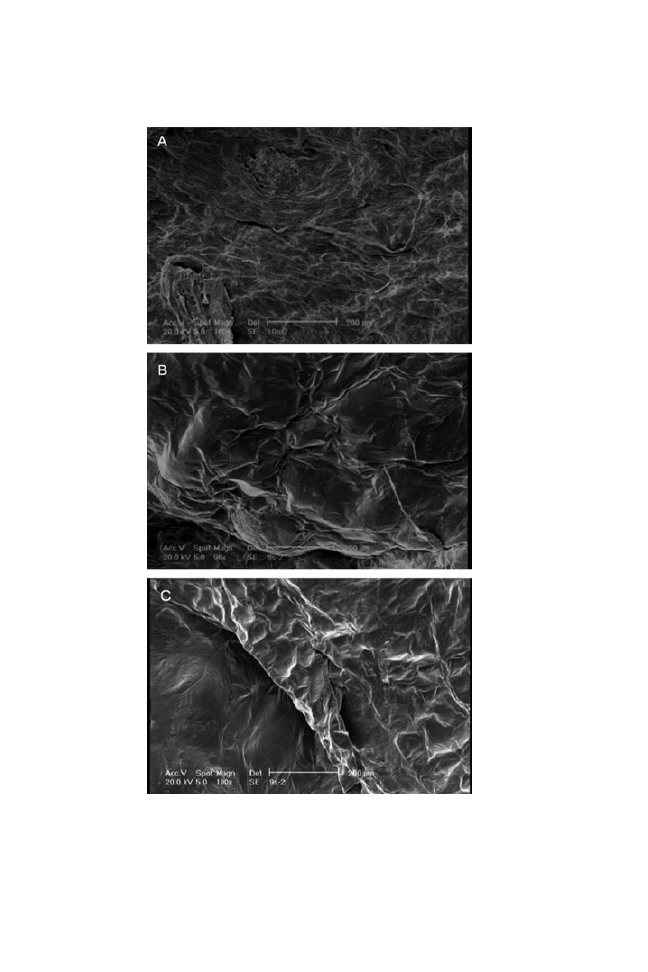
FIG. 8. SCANNING ELECTRON MICROSCOPY IMAGE OF FRESH (A) AND OSMOTIC
PRETREATED BY 5% NaCl
+ 40% SUCROSE (B) AND OSMOTIC PRETREATED BY
10% NaCl
+ 40% SUCROSE (C) TOMATO SAMPLES, AFTER VACUUM DRYING WITH
THE SAME MAGNIFICATION (100
¥)
384
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI
A
B
C
FIG. 9. SCANNING ELECTRON MICROSCOPY IMAGE OF FRESH (A) AND OSMOTIC
PRETREATED BY 5% NaCl
+ 40% SUCROSE (B) AND OSMOTIC PRETREATED BY
10% NaCl
+ 40% SUCROSE (C) TOMATO SAMPLES, AFTER HOT-AIR MICROWAVE
DRYING WITH THE SAME MAGNIFICATION (100
¥)
385
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES
greatly affected the samples. Because of the higher loss factor as a result of
higher salt content in samples pretreated with S
4
solution, greater surface
collapse was observed. At the end of the drying process, approximately all
open pores were case hardened; water vapor introduced by microwave energy
only affected the surface while crossing the superficial layer, so sample surface
collapse occurred. On the other hand, high water vapor pressure moved the
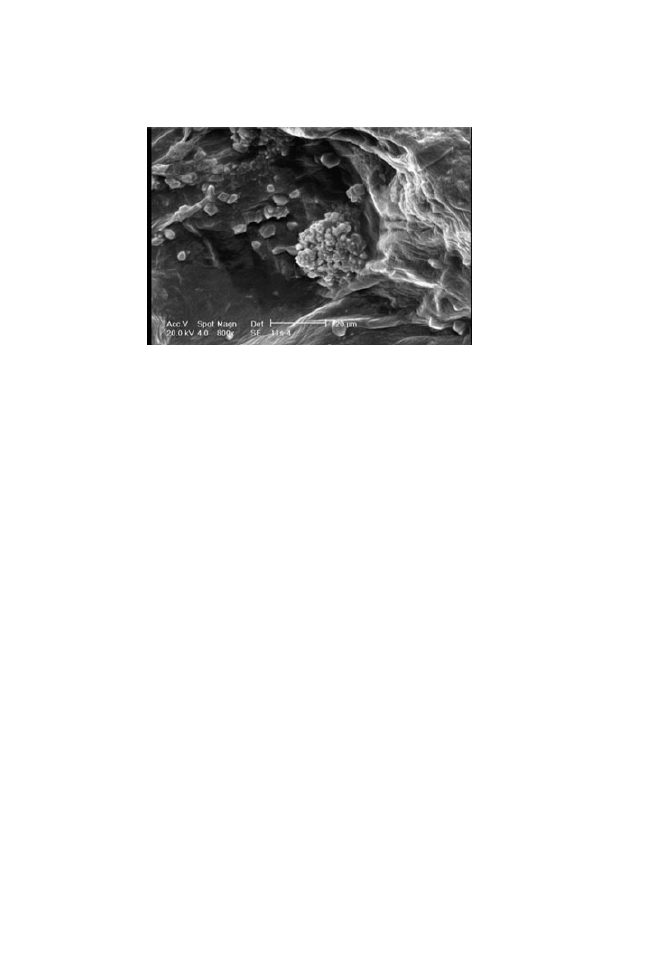
associated solute under the superficial layer to the outer parts. Figure 10 shows
the formation of solute crystals on the surface of the samples. Both S
3
and S
4
pretreated samples showed this behavior.
CONCLUSION
The effect of osmotic pretreatment in combination with hot-air, vacuum
and hot-air microwave drying methods on the progress of the drying process,
color and textual properties of tomato halves were investigated. Osmotic
pretreatment reduced drying time in all drying methods but it has more
effect on hot air drying (28% of time reduction) compared with other
methods. Results showed that using osmotic dehydration prior to drying
could preserve better the color of dried products. This effect is more con-
siderable when hot air drying or vaccum drying is used. Regarding hardness,
applying osmotic pretreatment prevents occurring textural hardness during
drying. Its effect is more evident in the case of hot air-microwave drying.
Applying osmotic dehydration in combination with hot air-microwave drying
FIG. 10. SCANNING ELECTRON MICROSCOPY IMAGE OF OSMOTIC PRETREATED BY
5% NaCl
+ 40% SUCROSE AND HOT-AIR MICROWAVE DRIED TOMATO WITH HIGHER
MAGNIFICATION (800
¥)
386
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI

decreases the hardness of dried tomatoes texture by 60%. Thus, osmotic
pretreatment reduces drying time and improves the texture and color of the
dried samples.
Among different drying methods, hot air-microwave drying seems to
be a more suitable method to preserve better the quality attributes of
dried tomatoes by reducing drying time and the harness of dried tomatoes
texture.
REFERENCES
ASKARI, G.R., EMAM-DJOMEH, Z. and MOUSAVI, S.M. 2006. Effects of
combined coating and microwave assisted hot-air drying on the texture,
microstructure and rehydration characteristics of apple slices. Food Sci.
Technol. Int. 12, 39–46.
ASSOCIATION OF OFFICIAL ANALYTICAL CHEMIST (AOAC) 1980.
Official Methods of Analysis. AOAC, Washington, DC.
AZOUBEL, P.M. and MURR, F.E. 2004. Mass transfer kinetics of osmotic
dehydration of cherry tomato. J. Food Eng. 61, 291–295.
CHIRLAT, A. and TALENS, P. 2005. Physical and chemical changes induced
by osmotic dehydration in plant tissue. J. Food Eng. 67, 167–177.
COLLINS, J.L., SIDHU, H.S. and MULLINS, C.A. 1997. Drying tomatoes
through osmotic treatment and dehydration. Tennessee Agric. Sci. 182,
24–27.
DE LOS REYES, R., HEREDIA, A., FITO, P., DE LOS REYES, E.
and ANDRES, E. 2007. Dielectric spectroscopy of osmotic solutions
and osmotically dehydrated tomato products. J. Food Eng. 80, 1218–
1225.
DROUZAS, A.E. and SCHUBERT, H. 1996. Microwave application in
vacuum drying of fruits. J. Food Eng. 28, 203–209.
DROUZAS, A.E., TSAMI, E. and SARAVACOS, G.D. 1999. Microwave/
vacuum drying of fruit gels. J. Food Eng. 39, 117–122.
ERTEKIN, F.K. and CAKALOZ, T. 1996. Osmotic dehydration of pears:
influence of process variables on mass transfer, J. Food Process. Preserv.
20, 87–104.
FENG, H. and TANG, J. 1998. Microwave finish drying diced apples in a
spouted bed. J. Food Sci. 63, 679–683.
GOULA, A.M., ADAMOPOULOS, K.G., CHATZITAKIS, P.C. and NIKAS,
V.A. 2006. Prediction of lycopene degradation during a drying process of
tomato pulp. J. Food Eng. 74, 37–46.
GUPTA, R.G. and NATH, N. 1984. Drying of tomatoes. J. Food Sci. Technol.
21, 372–376.
387
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES

HEREDIA, A., BARRERA, C. and ANDRES, A. 2007. Drying of cherry
tomato by a combination of different dehydration techniques: Compari-
sons of kinetics and other related properties. J. Food Eng. 80, 111–118.
INTERNATIONAL ELECTROTECHNICAL COMMISSION (IEC). 2004.
International Standard for Colorimetry, IEC 60214-2. IEC, Geneva,
Switzerland.
KHRAISHEH, M.A.M., COOPER, T.J.R. and MAGEE, T.R.A. 1997. Shrink-
age characteristics of potatoes dehydrated under combined microwave
and convective air conditions. Dry. Technol. 15, 1003–1022.
KOWALSKA, H. and LENART, A. 2001. Mass exchange during osmotic
pretreatment of vegetables. J. Food Eng. 49, 137–140.
LAZARIDES, H.N., KATSANIDIS, E. and NICKLADIS, A. 1995. Mass
transfer during osmotic preconcentration aiming at minimal solid uptake.
J. Food Eng. 25, 151–166.
LEWICKI, P.P., POMARANSKA-LAZUKA, W. and VU LE, H. 2002. Effect
of pre-treatment on convective drying of tomatoes. J. Food Eng. 54,
141–146.
MASKAN, M. 2001. Microwave/air and microwave finish drying of bananas.
J. Food Eng. 44, 71–78.
MORENO, J., CHIRALT, A., ESCRICHE, I. and SERRA, J.A. 2000. Effect of
blanching/osmotic dehydration combined methods on quality and stabil-
ity of minimally processed strawberries. Food Res. Int. 33, 609–616.
MUDGETT, R.E. 1989. Microwave food processing. Food Technol. 43, 117–
126.
MUJUMDAR, A.S. 2000. Drying Technology in Agricultural and Food
Science. Science Publishers, Inc., Plymouth, U.K.
PARK, K.J., BIN, A., BROD, F.P.R. and PARK, T.H.K.B. 2002. Osmotic
dehydration kinetics of pear D’anjou (Pyrus Communis L.). J. Food Eng.
52, 293–298.
PEREIRA, N.R., MARSAIOLI, JR., A. and AHRNE, L.M. 2007. Effect of
microwave power, air velocity and temperature on the final drying of
osmotically dehydrated bananas. J. Food Eng. 81, 79–87.
SHI, J.X., LE MAGUER, M., KAKUDA, Y., LIPTAY, A. and NIEKAP, F.
1999. Lycopene degradation and isomeration in tomato dehydration.
Food Res. Int. 32(1), 15–21.
SILVEIRA, E.T.F., RAHMAN, M.S.H. and BUCKLE, K.A. 1996. Osmotic
dehydration of pineapple: Kinetics and product quality. Food Res. Int.
29(3–4), 227–233.
STOJANOVIC, J. and SILVA, J.L. 2007. Influence of osmotic concentration,
continous high frequency ultrasound and dehydration on antioxidants,
colour and chemical properties of rabbiteye blueberries. Food Chem. 101,
898–906.
388
G.R. ASKARI, Z. EMAM-DJOMEH and M. TAHMASBI

TOOR, R.K. and SAVAGE, G.P. 2006. Effect of semi-drying on the antioxi-
dant components of tomatoes. Food Chem. 94, 90–97.
TORREGGIANI, D. 1993. Osmotic dehydration in fruits and vegetables. Food
Res. Int. 26, 59–68.
VALENCIA RODRIGUEZ, T., ROJAS, A.M., CAMPOS, C.A. and GER-
SCHENSON, L.N. 2003. Effect of osmotic dehydration on the quality of
air-dried Porphyra. Lebensm.-Wiss. Technol. 36, 415–422.
389
TEXTURE AND COLOR CHANGES IN DRYING TOMATOES

Wyszukiwarka
Podobne podstrony:
Influence of drying methods on drying of bell pepper (Tunde Akintunde, Afolabi, Akintunde)
Effect of vacuum microwave drying on selected mechanical and rheological properties of carrot
Microwave drying characteristics of potato and the effect of different microwave powers on the dried
Effect of Drying Techniques and Storage on Mulberry (Morus alba) Quality
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
31 411 423 Effect of EAF and ESR Technologies on the Yield of Alloying Elements
Effects of the Great?pression on the U S and the World
Possible Effects of Strategy Instruction on L1 and L2 Reading
76 1075 1088 The Effect of a Nitride Layer on the Texturability of Steels for Plastic Moulds
Effect of caffeine on fecundity egg laying capacity development time and longevity in Drosophila
Effect of Drugs and Alcohol on Teenagers
A systematic review and meta analysis of the effect of an ankle foot orthosis on gait biomechanics a
Effect of heat treatment on microstructure and mechanical properties of cold rolled C Mn Si TRIP
71 1021 1029 Effect of Electron Beam Treatment on the Structure and the Properties of Hard
53 755 765 Effect of Microstructural Homogenity on Mechanical and Thermal Fatique
EFFECTS OF EATING AND NOT EATING ON ENERGY STORES AND BODY WEIGHT
Glińska, Sława i inni The effect of EDTA and EDDS on lead uptake and localization in hydroponically
więcej podobnych podstron