Journal of Catalysis 224 (2004) 484–488
Research Note
Highly selective synthesis of menthols from citral in a one-step process
A.F. Trasarti, A.J. Marchi, and C.R. Apesteguía
∗
Catalysis Science and Engineering Research Group (GICIC), Instituto de Investigaciones en Catálisis y Petroquímica (INCAPE), UNL-CONICET,
Santiago del Estero 2654, (3000) Santa Fe, Argentina
Received 11 December 2003; revised 5 March 2004; accepted 10 March 2004
Abstract
We report for the first time the selective synthesis of menthols from citral in a one-step process. Bifunctional metal/acid catalysts active and
selective for menthol synthesis were developed by studying the individual steps involved in the reaction pathway leading to menthols from
citral. The metallic component was selected by testing silica-supported metals for citral hydrogenation to citronellal. Acid site requirements
to efficiently isomerize citronellal to isopulegols were investigated on different solid acids. Potential bifunctional metal/acid catalysts were
then prepared and tested for citral conversion to menthols. The best catalyst was Ni/Al-MCM-41, which yielded about 90% menthols and
gave 70–75% of racemic (
±)-menthol in the menthol mixture.
2004 Elsevier Inc. All rights reserved.
Keywords: Menthol synthesis; Citral conversion; Fine chemistry; Sustainable processes
1. Introduction
Menthol is used extensively in pharmaceuticals, cosmet-
ics, toothpastes, and chewing gum, as well as in cigarettes.
The menthol molecule comprises four pair of optical iso-
mers: (
±)-menthol, (±)-isomenthol, (±)-neomenthol, and
(
±)-neo-isomenthol. Of the eight optically active isomers,
only (
−)-menthol possesses the characteristic peppermint
odor and exerts a unique cooling sensation on the skin
and mucous membranes. Most (
−)-menthol is obtained by
freezing peppermint and cornmint oils, but it is also pro-
duced synthetically. In 1998, production of synthetic men-
thol was 2500 metric tons, which represented about 20%
of the world production of menthol [1]. Synthetic men-
thol is currently produced in the world by two companies:
Haarmann & Reimer and Takasago International Corpora-
tion. In the Haarmann & Reimer process [2] racemic (
±)-
menthols are obtained by hydrogenation of thymol, which
is, in turn, produced by propylation of m-cresol, a non-
stereospecific feedstock. (
−)-Menthol is obtained by further
treating the racemic menthol via a separation crystalliza-
tion process. This is the first commercial synthetic route
to (
−)-menthol. In the early 1980s, Takasago developed
an asymmetric synthesis technology for producing (
−)-
*
Corresponding author. Fax: 54-342-4531068.
E-mail address: capesteg@fiqus.unl.edu.ar (C.R. Apesteguía).
menthol from myrcene [3]. The key to the Takasago process
was the use of a chiral Rh BINAP catalyst for transform-
ing diethylgeranylamine obtained from myrcene to the chiral
3R-citronellal enamine, with more than 95% enantiomeric
excess.
Considerable effort has been devoted to the production
of (
−)-menthol by synthetic or semisynthetic means from
other more readily reliable raw materials. We report here for
the first time the selective synthesis of menthols from citral
in a one-step process, which involves the initial hydrogena-
tion of citral to citronellal, followed by the isomerization
of citronellal to isopulegols, and the final hydrogenation of
isopulegols to menthols. Menthols from citral is an attrac-
tive synthetic route because citral is a renewable raw mate-
rial that is obtained mainly by distillation of essential oils,
such as lemongrass oil, which contains ca. 70–80% citral.
Reactions involved in the citral-to-menthols pathway have
been studied separately. For example, the selective hydro-
genation of citral was widely investigated on different metal-
supported catalysts for producing either nerol/geraniol or
citronellal and citronelol [4,5]. The citronellal cyclization
to isopulegol has been carried out by using liquid [6,7]
and solid [8–10] acid catalysts, while the direct synthesis
of menthols from citronellal was recently investigated on
ruthenium-based catalysts [11]. In this note we report the
development of bifunctional metal–acid catalysts that pro-
mote selectively the direct conversion of citral to menthols.
0021-9517/$ – see front matter
2004 Elsevier Inc. All rights reserved.
doi:10.1016/j.jcat.2004.03.016

A.F. Trasarti et al. / Journal of Catalysis 224 (2004) 484–488
485
The best catalyst was Ni supported on Al-MCM-41, which
yields about 90% menthols from citral and gives 70–75%
racemic (
±)-menthol in the menthol mixture.
2. Experimental
Six catalysts were prepared by supporting metals
[Pt(0.3%), Pd(0.7%), Ir(1%), Ni(12%), Co(12%), Cu(12%)]
on a SiO
2
powder (Grace G62, 99.7%). The silica has
a BET surface area (S
g
) of 230 m
2
/g and pore volume
of 0.49 cm
3
/g. Pt/SiO
2
catalyst was made by incipient-
wetness impregnation at 303 K with an aqueous solution
of tetraamine platinum nitrate, Pt(NH
3
)
4
(NO
3
)
2
(Alfa). The
impregnated silica was dried overnight at 363 K, then heated
in N
2
to 673 K at 2 K/min, and finally reduced for 2 h at
673 K in pure hydrogen. The other five metal/SiO
2
catalysts
were prepared following the same preparation procedure of
Pt/SiO
2
. Metal nitrate solutions were used for impregnating
Pd, Co, Ni, and Cu, whereas Ir/SiO
2
was prepared by using
H
2
IrCl
6
precursor.
Al-MCM-41 (Si/Al
= 10) was synthesized by the sol–
gel method. Sodium silicate solution (14% NaOH and
27% SiO
2
, Aldrich), cetyltrimethylammonium bromide
(Aldrich), aluminum isopropoxide, and deionized water
were used as the reagents. The composition of the synthesis
gel was 7SiO
2
–xAl
2
O
3
–2.7Na
2
O–3.7CTMABr–1000H
2
O.
The pH was adjusted to 10 using a 1 M H
2
SO
4
solution,
then the gel was transferred to a Teflon-lined stainless-
steel autoclave and heated to 373 K in an oven for 96 h.
After crystallization, the solid was washed with deionized
water, dried at 373 K, and finally calcined at 773 K for
4 h. Al-MCM-41 has a BET surface area of 690 m
2
/g,
and mean pore diameter of 30 Å. Zeolite Beta (Zeocat PB,
Si/Al
= 25, S
g
= 630 m
2
/g) was calcined in air at 773 K
for 4 h. ZnO(25%)/SiO
2
was made by incipient-wetness
impregnation at 303 K of Grace G62 SiO
2
with an aque-
ous solution of ZnCl
2
(Riedel de Haën, 98.5%). Cs-HPA
(Cs
0.5
H
2.5
PW
12
O
40
, S
g
= 130 m
2
/g) was prepared using
H
3
PW
12
O
40
· 6H
2
O (HPA, Merck p.a.) and CsCO
3
(Sigma).
Cs-HPA was obtained by precipitation, by adding dropwise
a solution of CsCO
3
to an aqueous solution of HPA. Nickel
supported on zeolite Beta (3% Ni/Beta) and nickel sup-
ported on Al-MCM-41 (3% Ni/Al-MCM-41) were obtained
by incipient-wetness impregnation at 303 K, using Ni(NO
3
)
2
(Alfa). The impregnated samples were dried overnight at
363 K, then treated in air at 673 K for 6 h and reduced in H
2
for 2 h at 723 K.
Acid site densities were determined by temperature pro-
grammed desorption (TPD) of NH
3
preadsorbed at 373 K.
Samples (0.200 g) were treated in He (60 ml/min) at 773 K
for 0.5 h and then exposed to a 1% NH
3
/He stream for
40 min at 373 K. Weakly adsorbed NH
3
was removed by
flowing He at 373 K during 2 h. Temperature was then
increased at 10 K/min, and the NH
3
concentration in the
effluent was measured by mass spectrometry in a Baltzers
Omnistar unit.
The nature of surface acid sites was determined by in-
frared spectroscopy (IR) using pyridine as probe molecule.
Data were obtained using a Shimadzu FTIR-8101M spec-
trophotometer after admission of pyridine, adsorption at
room temperature, and sequential evacuation at 298, 423,
573, and 723 K. Spectra were taken at room temperature.
The crystalline structure of the samples was determined by
X-ray diffraction (XRD) using a Shimadzu XD-D1 diffrac-
tometer. BET surface areas (S
g
) were measured by N
2
ph-
ysisorption at its boiling point in a Quantochrome Corpora-
tion NOVA-1000 sorptometer.
The liquid phase hydrogenation of citral (Aldrich, 98%,
isomer mixture of 42% cis and 58% trans) was studied in
a Parr 4843 reactor at 343 and 393 K, using isopropanol or
toluene (Cicarelli, p.a.) as solvent. The autoclave was loaded
with 150 ml of solvent, 10 ml of citral, and 0.2–1 g of cata-
lyst. Prior to catalytic tests, samples were activated ex situ
in flowing hydrogen (30 ml/min) at 473 K for 1 h. The
reaction system was heated to the reaction temperature of
2 K/min, and the pressure was then rapidly increased to
506.5 or 1013 kPa with H
2
. The liquid phase conversion of
citronellal (Sigma, 95%) was carried out in the same reactor
used for citral hydrogenation. The reactor was loaded with
150 ml of toluene, 2 ml of citronellal, and 0.200 g of cata-
lyst. The reaction was performed at 343 K and 506.5 kPa
of nitrogen. Product concentrations were followed during
the reaction by ex situ gas chromatography using an Agilent
6850 GC chromatograph equipped with flame ionization de-
tector, temperature programmer, and a 30-m Innowax (Ag-
ilent) column coupled with a 30-m Supelco α-DEX capil-
lary column. Data were collected every 15–30 min for about
500 min. Selectivities (S
j
, moles of product j/moles of cit-
ral reacted) were calculated as S
j
(%)
= C
j
× 100/
C
j
,
where C
j
is the concentration of product j . Product yields
(η
j
, moles of product j/moles of citral fed) were calculated
as η
j
= S
j
X
Cit
.
3. Results and discussion
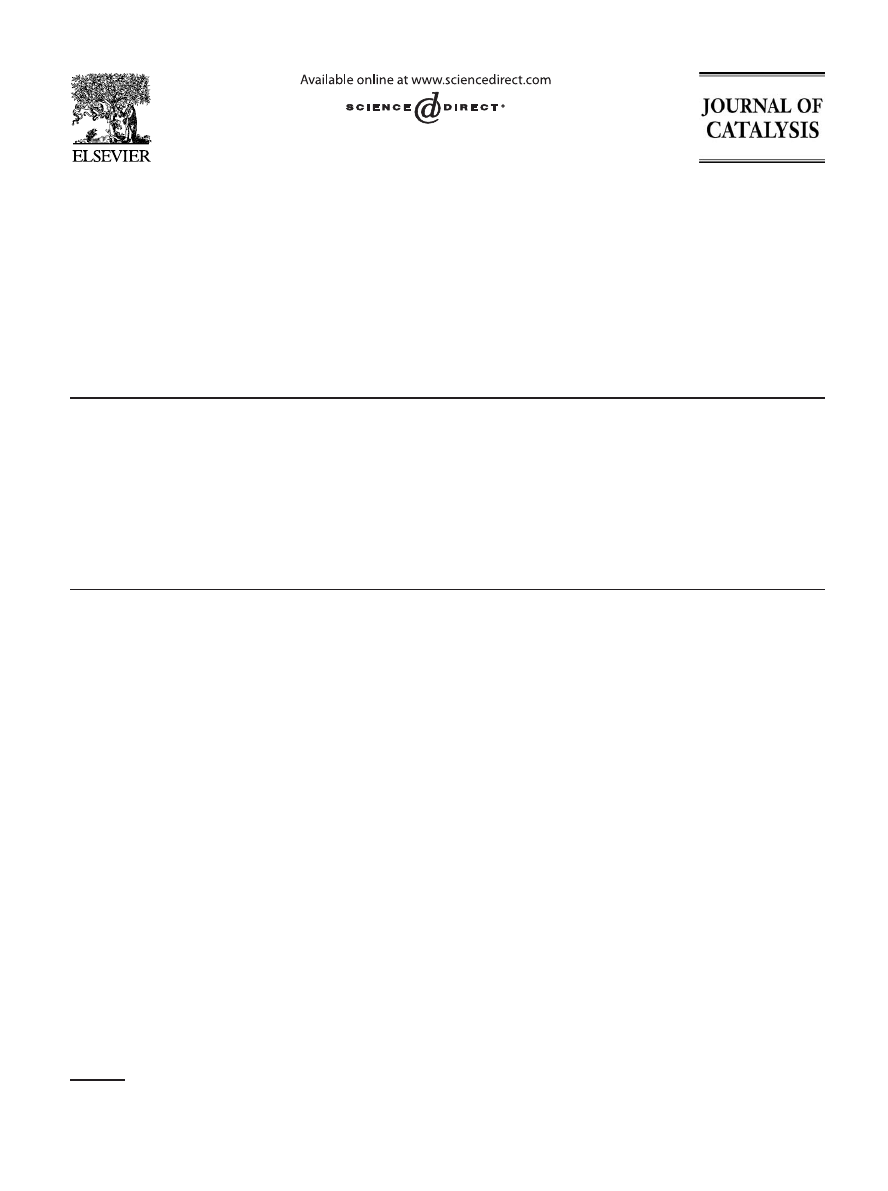
Fig. 1 shows the citral conversion reaction network. The
reaction pathway leading from citral to menthols involves
three consecutive steps: (i) hydrogenation of citral to cit-
ronellal; (ii) isomerization/cyclization of citronellal to isop-
ulegol; (iii) hydrogenation of isopulegol to menthols. Fig. 1
shows that the selective transformation of citral to menthols
requires bifunctional catalysts with the ability not only of
promoting coupled hydrogenation/isomerization reactions
of the citral-to-menthols pathway but also of minimizing the
parallel hydrogenation reactions of citral to nerol/geraniol or
3,7-dimethyl-2,3-octenal and of citronellal to citronelol or
3,7-dimethyloctanal. In other words, from a kinetic point of
view the selective formation of menthols from citral requires
that k
1
(k
4
+ k
5
) and k
2
(k
6
+ k
7
) (Fig. 1).
486
A.F. Trasarti et al. / Journal of Catalysis 224 (2004) 484–488
Fig. 1. Reaction network for citral conversion reactions.
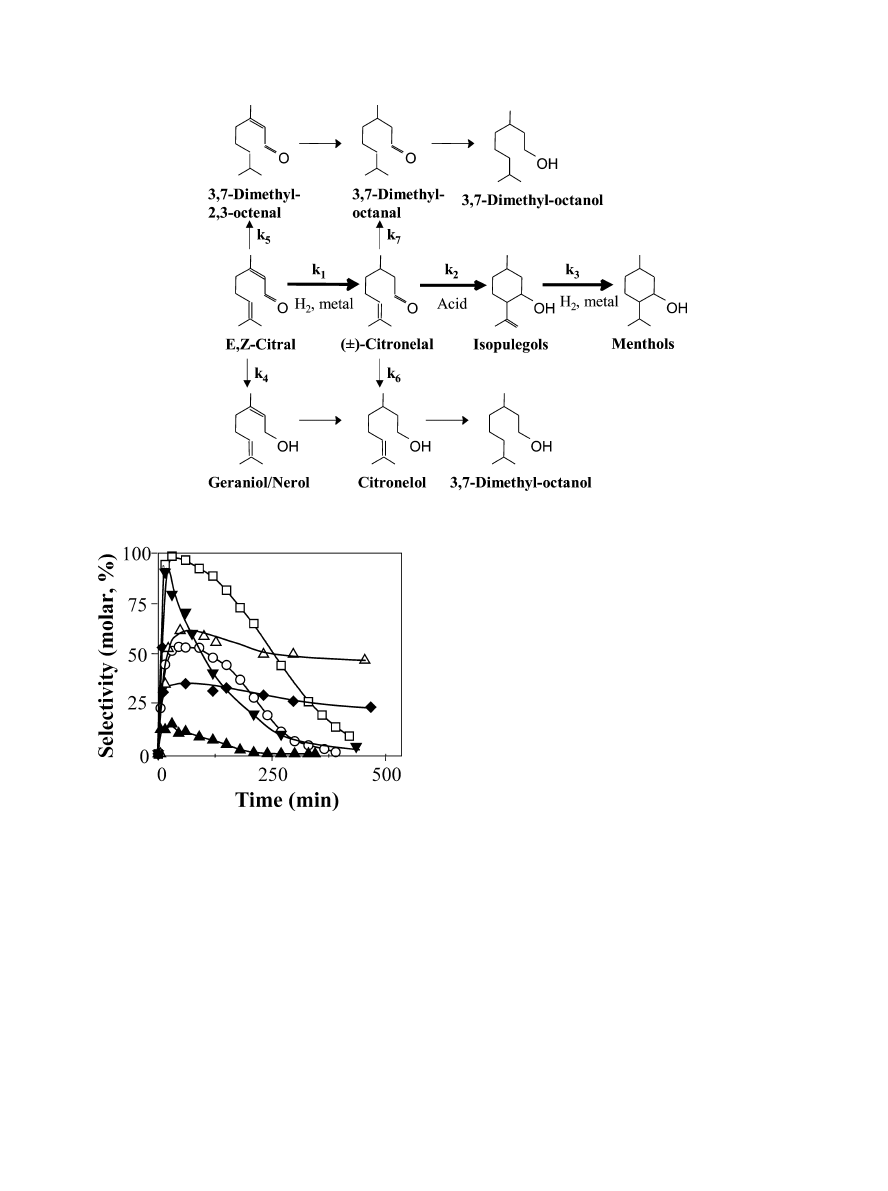
Fig. 2. Hydrogenation of citral: selectivities toward citronellal as a func-
tion of time. Ni/SiO
2
(1), Pd/SiO
2
(a), Pt/SiO
2
(P), Cu/SiO
2
(!),
Ir/SiO
2
(F), Co/SiO
2
(Q) [393 K, 1013 kPa hydrogen, W = 1 g,
citral:isopropanol
= 2:150 (ml)].
We first investigated the initial hydrogenation of citral
by comparing the catalytic performance of different silica-
supported metals. Samples were tested at 393 K using as sol-
vent isopropanol, a polar compound. Only products formed
via citral hydrogenation reactions were observed; isopule-
gol was never detected, reflecting the absence of surface
acid sites on the support. In Fig. 2 we have plotted cit-
ronellal selectivity (S
j
, moles of product j/moles of citral
reacted) as a function of reaction time. Pd/SiO
2
and Ni/SiO
2
selectively hydrogenated the conjugated C
=C bond of the
citral molecule, forming initially citronellal in about 100%
selectivity. The citronellal selectivity decreases then with re-
action time because citronellal is in turn hydrogenated to
citronelol or 3,7-dimethyloctanal. But it should be noted
that on Pd and Ni metals, it is verified that k
1
(k
4
+ k
5
).
In contrast, Co/SiO
2
and, to a lesser degree, Ir/SiO
2
pref-
erentially promoted the initial hydrogenation of citral to
nerol/geraniol isomers, and thus citronellal was a minor
product. On Co/SiO
2
the maximum citronellal selectivity
was lower than 20%. Finally, on Cu/SiO
2
and Pt/SiO
2
the
citral conversion rates to citronellal and to geraniol/nerol
were similar, and thereby the initial citronellal selectivity
was about 50–60% on both catalysts. Overall, our results
are consistent with previous works on citral hydrogenation
showing that Ni and Pd favor C
=C bond hydrogenation [4]
while Co and Ir are more selective for C
=O hydrogena-
tion [5].
In a second part, we studied the isomerization of cit-
ronellal to isopulegols. Cyclization of citronellal has been
investigated on different solid acids, but the exact nature
of the surface active sites required for efficiently catalyzing
the cyclization of citronellal to isopulegols is still debated.
While several authors [9] reported that the reaction is read-
ily catalyzed on Lewis acids, others [8] correlated the cy-
clization activity on acid zeolites with accessible Brønsted
acid sites. Chuah et al. [10] found that catalytic materials
containing strong Lewis and weak Brønsted acidity show
good activity and selectivity for cyclization of citronellal
to isopulegol. They proposed then a cyclization mechanism
based on the coordination of the citronellal to a strong Lewis
site, followed by protonation from a Brønsted acid site. In
this work we used two solid acids containing either Lewis

A.F. Trasarti et al. / Journal of Catalysis 224 (2004) 484–488
487
(ZnO/SiO
2
) or Brønsted (Cs-HPA) sites and two catalysts
containing weak (Al-MCM-41) and strong (zeolite Beta)
Lewis and Brønsted acid sites. In all the cases, isopulegol
isomers were the only products detected. We found that the
citronellal cyclization rate is clearly lower on ZnO/SiO
2
and
Cs-HPA samples as compared with both zeolite Beta and
Al-MCM-41. The superior activity shown by zeolite Beta
and Al-MCM-41 samples are consistent with the assumption
that strong Lewis/weak Brønsted dual sites are required to
efficiently catalyze the citronellal cyclization. We therefore
performed a detailed characterization of the surface acid site
nature and strength on zeolite Beta and Al-MCM-41 sam-
ples.
The NH
3
TPD profiles showed that on Al-MCM-41, NH
3
desorbs in a band between 425 and 550 K while on ze-
olite Beta NH
3
desorbs in two broad bands, from 425 to
800 K. The NH
3
surface densities for acid sites on both sam-
ples were obtained by deconvolution and integration of TPD
traces. It was determined that the total adsorbed NH
3
surface
density on zeolite Beta (0.92 µmol/m
2
) was significantly
higher compared with that on Al-MCM-41 (0.20 µmol/m
2
).
On the other hand, from the IR spectra of adsorbed pyri-
dine at 373 K we measured the areal densities of Lewis and
Brønsted acid sites and found that the Lewis/Brønsted site
ratios were about 1 and 2.5 on zeolite Beta and Al-MCM-
41, respectively. The Brønsted and Lewis site densities per
square meter on zeolite Beta were about seven and two times
higher, respectively, than on Al-MCM-41. In summary, sam-
ple acidity characterization revealed that zeolite Beta con-
tains a higher density of stronger acid sites compared with
Al-MCM-41.
Based on the above results, we prepared three bifunc-
tional catalysts containing one of the metals most selec-
tive for hydrogenating citral to citronellal (Pd or Ni) and
one of the solid acids more active for converting citronel-
lal to isopulegols (zeolite Beta or Al-MCM-41). Specifically,
we prepared Pd (1%) supported on zeolite Beta (Pd/Beta)
and Ni (3%) supported on zeolite Beta (Ni/Beta) and on
Al-MCM-41 (Ni/Al-MCM-41). These bifunctional catalysts
were tested for the conversion of citral to menthols at 343 K
and P
H
2
= 506.5 kPa, using 1 g of sample and toluene as
solvent. Catalytic results are given in Table 1. In all the
cases, citral and citronellal were totally converted after 5 h
of reaction. Pd/Beta produced a considerable amount of 3,7-
dimethyl-octanal, thereby showing that Pd is significantly
active for hydrogenating the C
=C bond of citronellal. Thus,
it seems that the second requirement for selectively obtain-
ing menthols from citral [i.e., k
2
(k
6
+ k
7
)] (Fig. 1) is not
fulfilled using Pd as the metal component of the bifunctional
catalyst because of the high value of rate constant k
7
on Pd.
As shown in Table 1, the yield of menthols was only about
20% on Pd/Beta catalyst. Ni/Beta was clearly more selec-
tive to menthols than Pd/Beta, essentially because formation
of 3,7-dimethyl-octanal was negligible on Ni/Beta. Actu-
ally, none of the by-products formed from hydrogenation
of citral or citronellal (Fig. 1) were detected using Ni/Beta,
suggesting that this bifunctional catalyst satisfactorily com-
bines the hydrogenation and isomerization functions needed
to selectively promote the reaction pathway leading from
citral to menthols. However, formation of secondary com-
pounds formed probably via decarbonylation and cracking
reactions on the strong acid sites of zeolite Beta was sig-
nificant, and the by-product yield on Ni/Beta was about
20%. The best catalyst was Ni/Al-MCM-41, which yielded
ca. 90% menthols. The observed menthol yield improve-
ment on Ni/Al-MCM-41 is probably explained by consid-
ering that the moderate acid sites of Al-MCM-41 do not
promote the formation of by-products via side cracking reac-
tions. In contrast, Al-MCM-41 rapidly isomerizes citronellal
to isopulegols, and menthols are then formed at high rates.
Regarding the distribution of menthol isomers, it is noted
that (
±)-neo-isomenthol was never detected in the prod-
ucts. On Ni-based catalysts, the menthol mixture was com-
posed of 70–75% (
±)-menthols, 15–20% (±)-neo-menthol,
and 5–10% (
±)-isomenthol. On Pd/Beta the racemic (±)-
menthol mixture represented only about 50% of total men-
thols.
The evolution of product yields and citral conversion as a
function of time on Ni/Al-MCM-41 (not shown here) indi-
cated that citral was totally converted to citronellal on metal-
lic Ni crystallites in about 80 min, but the concentration of
citronellal remained very low (maximum citronellal yield
was about 10% at 25 min) because it was readily converted
to isopulegols on acid sites of mesoporous Al-MCM-41 sup-
port. Isopulegols were then totally hydrogenated to menthols
on metal Ni surface sites.
In summary, Ni(3%)/Al-MCM-41 yields 90% menthols
directly from citral and produces 70–75% racemic (
±)-
menthol in the menthol mixture. Further improvement in
catalytic performance is certainly expected by optimizing
both the catalyst formulation (Ni loading, Si/Al ratio) and
the reactor operation (hydrogen pressure).
Table 1
Product yields and menthol isomer distribution
a
Catalyst
Yield (%)
b
Menthol isomer distribution (%)
b
Menthols
3,7-DMAL
Isopulegols
Others
(
±)-Menthols
(
±)-Neomenthol
(
±)-Isomenthol
Pd/Beta
22.0
25.8
18.5
33.7
47.2
15.6
37.2
Ni(3%)/Beta
81.0
0
0
19.0
72.0
21.3
6.7
Ni(3%)/Al-MCM-41
89.2
0
0
10.8
72.3
20.2
7.5
a
T
= 343 K, P
H
2
= 506.5 kPa, W = 1 g, citral:toluene = 2:150 (ml).
b
Values determined at 300 min reaction; X
cit
= 100% on all catalysts.

488
A.F. Trasarti et al. / Journal of Catalysis 224 (2004) 484–488
Acknowledgments
We thank the Universidad Nacional del Litoral (UNL),
Consejo Nacional de Investigaciones Científicas y Técnicas
(CONICET), and Agencia Nacional de Promoción Científica
y Tecnológica (ANPCyT), Argentina, for financial support
of this work.
References
[1] G.S. Clark, Menthol Perfumer Flavorist 25 (1998) 33.
[2] J. Fleischer, K. Bauer, R. Hopp, DE 2109456 (1971), Harrmann &
Reimer.
[3] S. Otsuka, K. Tani, T. Yamagata, S. Akutagawa, H. Kumobayashi,
M. Yagi, EP 68506 (1982), Takasago.
[4] P. Maki-Arvela, L.P. Tiainen, A.K. Neyestanaki, R. Sjöholm, T.K.
Rantakylä, E. Laine, T. Salmi, D.Yu. Murzin, Appl. Catal. A 237
(2002) 181.
[5] U.K. Singh, M.A. Vannice, J. Catal. 199 (2001) 73.
[6] V.K. Aggarwal, G.P. Vennall, C. Newman, Tetrahedron Lett. 39 (1998)
1997.
[7] P. Kocovský, G. Ahmed, A.V. Malkov, J. Steele, J. Org. Chem. 64
(1999) 2765.
[8] M. Fuentes, J. Magraner, C. De Las Pozas, R. Roquemalherbe, J. Perez
Pariente, A. Corma, Appl. Catal. 47 (1989) 367.
[9] C. Milone, A. Perri, A. Pistone, G. Neri, A. Pistone, S. Galvagno,
Appl. Catal. A 233 (2002) 151.
[10] G.K. Chuah, S.H. Liu, S. Jaenicke, L.J. Harrison, J. Catal. 200 (2001)
351.
[11] C. Milone, C. Gangemi, R. Ingoglia, G. Neri, S. Galvagno, Appl.
Catal. A 184 (2000) 89.
Document Outline
Wyszukiwarka
Podobne podstrony:
Isolation of lycopene from crude tomato extract via selective inclusion in deoxycholic acid
Morimoto, Iida, Sakagami The role of refections from behind the listener in spatial reflection
Morimoto, Iida, Sakagami The role of refections from behind the listener in spatial reflection
Validation of a test battery for the selection of call centre operators in a communications company
From Small Beginnings; The Euthanasia of Children with Disabilities in Nazi Germany
Fishea And Robeb The Impact Of Illegal Insider Trading In Dealer And Specialist Markets Evidence Fr
Selective Functionalization of Amino Acids in Water
an alternative and simple preparation of tryptamine from l tryptophan by catalytic decarboxylation w
Detection of Measles Virus RNA in Urine Specimens from Vaccine Recipients
Clive Staples Lewis The Grand Miracle; And Other Selected Essays On Theology And Ethics From God In
FROM VIPASSANA IN THERAVADA TO compaarative study of the moeditative techniques
DIMENSIONS OF INTEGRATION MIGRANT YOUTH IN POLAND
The?uses of the Showa Restoration in Japan
There are a lot of popular culture references in the show
Comparative Study of Blood Lead Levels in Uruguayan
Effect of?renaline on survival in out of hospital?rdiac arrest
Capability of high pressure cooling in the turning of surface hardened piston rods
więcej podobnych podstron