E X A M P L E 6 . 6
Entropy Production in a Steam Turbine
Steam enters a turbine with a pressure of 30 bar, a temperature of 400
C, and a velocity of 160 m/s. Saturated vapor at 100C
exits with a velocity of 100 m /s. At steady state, the turbine develops work equal to 540 kJ per kg of steam flowing through
the turbine. Heat transfer between the turbine and its surroundings occurs at an average outer surface temperature of 350 K.
Determine the rate at which entropy is produced within the turbine per kg of steam flowing, in
Neglect the change
in potential energy between inlet and exit.
S O L U T I O N
Known:
Steam expands through a turbine at steady state for which data are provided.
Find:
Determine the rate of entropy production per kg of steam flowing.
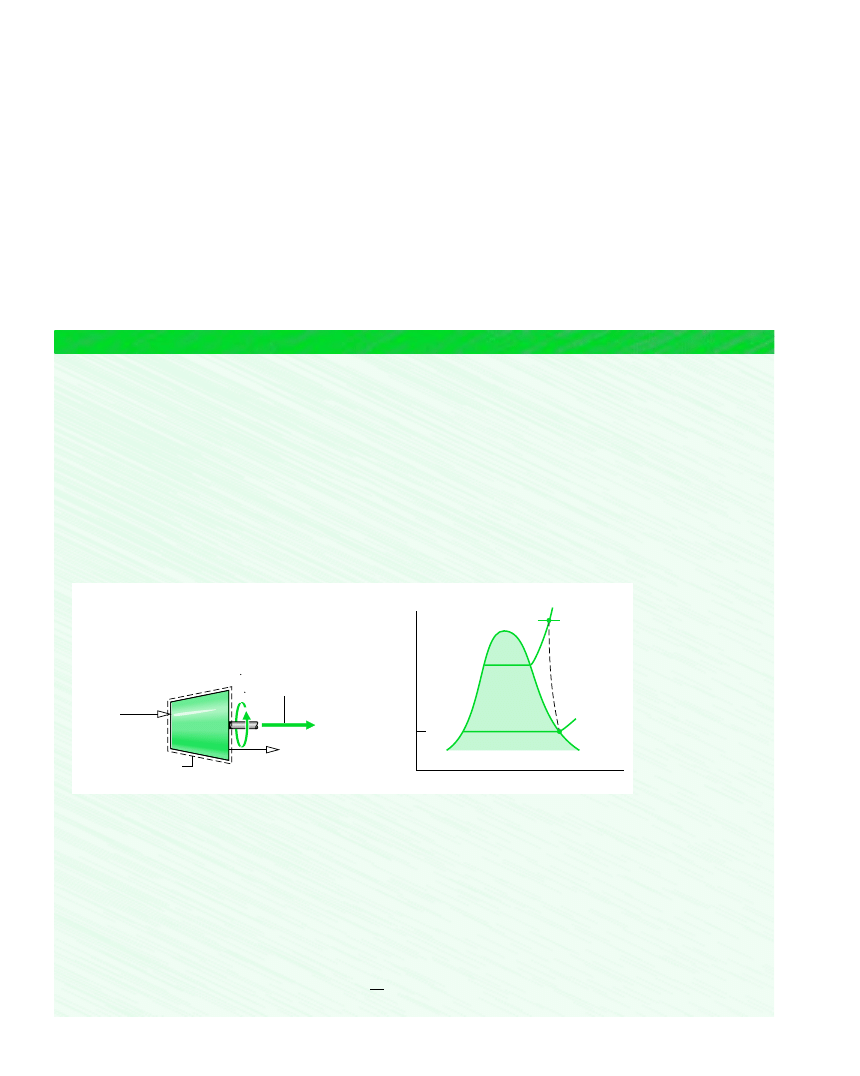
Schematic and Given Data:
kJ/kg
# K.
234
Chapter 6 Using Entropy
Accordingly, when irreversibilities are present within the control volume, the entropy of a
unit of mass increases as it passes from inlet to exit. In the limiting case in which no irre-
versibilities are present, the unit mass passes through the control volume with no change in
its entropy—that is, isentropically.
6.6.2
Illustrations
The following examples illustrate the use of the mass, energy, and entropy balances for the
analysis of control volumes at steady state. Carefully note that property relations and prop-
erty diagrams also play important roles in arriving at solutions.
In the first example, we evaluate the rate of entropy production within a turbine operat-
ing at steady state when there is heat transfer from the turbine.
Assumptions:
1.
The control volume shown on the accompanying sketch is at steady state.
2.
Heat transfer from the turbine to the surroundings occurs at a specified average outer surface temperature.
3.
The change in potential energy between inlet and exit can be neglected.
Analysis:
To determine the entropy production per unit mass flowing through the turbine, begin with mass and entropy rate
balances for the one-inlet, one-exit control volume at steady state:
0
a
j
Q
#
j
T
j
m
#
1
s
1
m
#
2
s
2
s
#
cv
0
m
#
1
m
#
2
Figure E6.6
1
2
p
1
T
1
V
1
= 30 bar
= 400
°C
= 160 m/s
T
2
= 100
°C
Saturated vapor
V
2
= 100 m/s
W
cv
–––
m
= 540 kJ/kg
T
b
= 350 K
1
2
400
°C
30 bar
T
100
°C
s

Since heat transfer occurs only at T
b
350 K, the first term on the right side of the entropy rate balance reduces to
.
Combining the mass and entropy rate balances
where
is the mass flow rate. Solving for
The heat transfer rate,
, required by this expression is evaluated next.
Reduction of the mass and energy rate balances results in
where the potential energy change from inlet to exit is dropped by assumption 3. From Table A-4 at 30 bar, 400
C, h
1
3230.9 kJ/kg, and from Table A-2, h
2
h
g
(100
C) 2676.1 kJ/kg. Thus
From Table A-2, s
2
and from Table A-4, s
1
Inserting values into the expression
for entropy production
If the boundary were located to include a portion of the immediate surroundings so heat transfer would take place at the
temperature of the surroundings, say T
f
293 K, the entropy production for the enlarged control volume would be
It is left as an exercise to verify this value and to explain why the entropy production for the enlarged
control volume would be greater than for a control volume consisting of the turbine only.
0.511 kJ/kg
# K.
0.0646 0.4337 0.4983
kJ/kg
# K
s
#
cv
m
#
122.6 kJ/kg2
350 K
17.3549 6.92122
a
kJ
kg
# K
b
6.9212 kJ/kg
# K.
7.3549 kJ/kg
# K,
540 554.8 7.8 22.6 kJ/kg
Q
#
cv
m
#
540
kJ
kg
12676.1 3230.92
a
kJ
kg
b c
11002
2
11602
2
2
d
a
m
2
s
2
b
`
1
N
1
kg
# m /s
2
`
`
1
kJ
10
3
N
# m
`
Q
#
cv
m
#
W
#
cv
m
#
1h
2
h
1
2 a
V
2
2
V
2
1
2
b
Q
#
cv
m
#
s
#
cv
m
#
Q
#
cv
m
#
T
b
1s
2
s
1
2
s
#
cv
m
#
m
#
0
Q
#
cv
T
b
m
# 1s
1
s
2
2 s
#
cv
Q
#
cv
T
b
In Example 6.7, the mass, energy, and entropy rate balances are used to evaluate a per-
formance claim for a device to produce hot and cold streams of air from a single stream of
air at an intermediate temperature.
E X A M P L E 6 . 7
Evaluating a Performance Claim
An inventor claims to have developed a device requiring no energy transfer by work or heat transfer, yet able to produce hot
and cold streams of air from a single stream of air at an intermediate temperature. The inventor provides steady-state test data
indicating that when air enters at a temperature of 39
C and a pressure of 5.0 bars, separate streams of air exit at temperatures
of
18C and 79C, respectively, and each at a pressure of 1 bar. Sixty percent of the mass entering the device exits at the lower
temperature. Evaluate the inventor’s claim, employing the ideal gas model for air and ignoring changes in the kinetic and po-
tential energies of the streams from inlet to exit.
❶
❶
6.6 Entropy Rate Balance for Control Volumes
235
Wyszukiwarka
Podobne podstrony:
Moran,Shapiro Fundamentals of Engineering Thermodynamics SI Version 5th Ed 252 255
Moran,Shapiro Fundamentals of Engineering Thermodynamics SI Version 5th Ed 311 313
Biotransformation of Tryptamine in Fruiting Mycelia of Psilocybe cubensis Planta Med (1989) 55 (3) 2
Biotransformation of Tryptamine in Fruiting Mycelia of Psilocybe cubensis Planta Med (1989) 55 (3) 2
Engineering Fundamentals of Digital Electronics
Fundamentals of Polymer Chemist Nieznany
Engineering Thermoplastics, Overview
Fundamentals of Zen Meditation
HRMH%20Rules%20of%20Thumb%20Edition%203%20Web%20Version
Fundamnentals of dosimetry based on absorbed dose standards
Fundamentals of Therapy
Fundamentals of Fluid Flow
Fundamentals of radiation dosimetry and radiological physics
Fundamentals of Project Management 4th ed J Heagney (AMACOM, 2012)
Pomiar prTź¦ůnoci pary nasyconej SI VERSION
Belyaev Fundamentals of Geometry
więcej podobnych podstron