Chemical Behaviour of Red Phosphorus in Water
OH
H
H
3
PO
2
+
X
H
3
PO
3
H
3
PO
4
X
Prepared by:
Clariant GmbH
BU Additives – Flame Retardants
65840 Sulzbach
Germany
Contact:
Dr. Rüdiger Walz
Tel: +49 6196 757 8109
Fax: +49 6196 757 8977
e-mail: ruediger.walz@clariant.com
Dr. Adrian Beard
Tel: +49 2233 48-6114
Fax: +49 2233 41236
e-mail: adrian.beard@clariant.com
14. January 2000

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
2
P-red-AquaChem-06.doc - 14.01.00
Contents
1
Summary
3
2
Technical red phosphorus
4
2.1
Economic background
4
2.2
Red phosphorus: short process description
6
2.3
Characteristics of red phosphorus in comparison with white
phosphorus
8
2.4
Content of white phosphorus in red phosphorus
10
3
Solubility of white phosphorus in water and related
reactions
11
4
Solubility of red phosphorus in water and related
reactions
12
4.1
Theoretical approach
12
4.2
Experimental data
13
4.2.1
Phosphorus-containing acids
13
4.2.2
No release of white phosphorus from red phosphorus in water
15
4.3
Reaction rate of red phosphorus with water
16
List of Abbreviations
calc.
calculated
d.l.
detection limit
EC 50
median effect concentration value
NMR
Nuclear magnetic resonance spectroscopy

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
3
P-red-AquaChem-06.doc - 14.01.00
1
Summary
This report describes the chemical behaviour of red phosphorus in water and compares it to
the reactions of white phosphorus. Whereas white phosphorus consists of P
4
molecules,
spontaneously ignites near room temperature and is highly toxic, red phosphorus is an allo-
tropic modification of elemental phosphorus which is mostly an amorphous polymer so that
the physico-chemical properties of red phosphorus are very different from white phospho-
rus. Red phosphorus ignites only above 260 °C, is of very low toxicity and moderate reac-
tivity.
Even white phosphorus has a very low solubility in water of about 3 mg/L. Due to its poly-
meric nature, red phosphorus is practically insoluble in water. However, since elemental
phosphorus is thermodynamically unstable in the presence of water and oxygen, red phos-
phorus will very slowly react via intermediates such as hypophosphorous acid (H
3
PO
2
),
phosphorous acid (H
3
PO
3
), and phosphine (PH
3
) to phosphoric acid (H
3
PO
4
). These reaction
products dissolve in water and contribute to a concentration of total phosphorus com-
pounds (calculated as phosphorus) of 1 mg/L after 24 hours starting from 100 mg/L. Even
after 4 months and starting from 10 000 mg/L the concentration of phosphorus compounds
reaches only 270 mg/L corresponding to a conversion rate of the red phosphorus of only
2.7 % (see Table 3 and Table 4). Since these disproportionating and hyrolysis reactions
proceed at a very slow rate, even critical products like phosphine (not readily soluble in wa-
ter) will be finally converted to phosphoric acid in oxygen containing environments.
Experiments indicate that the traces of white phosphorus (< 200 mg/kg) present as a con-
taminant in red phosphorus cannot be readily extracted by water. As a consequence, the
new and so far unpublished experimental data in this report indicate that the content of
white phosphorus in red phosphorus should not be used as a sole reference for extrapolat-
ing the toxic effects of red phosphorus to aquatic organisms.
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
4
P-red-AquaChem-06.doc - 14.01.00
2
Technical red phosphorus
2.1
Economic background
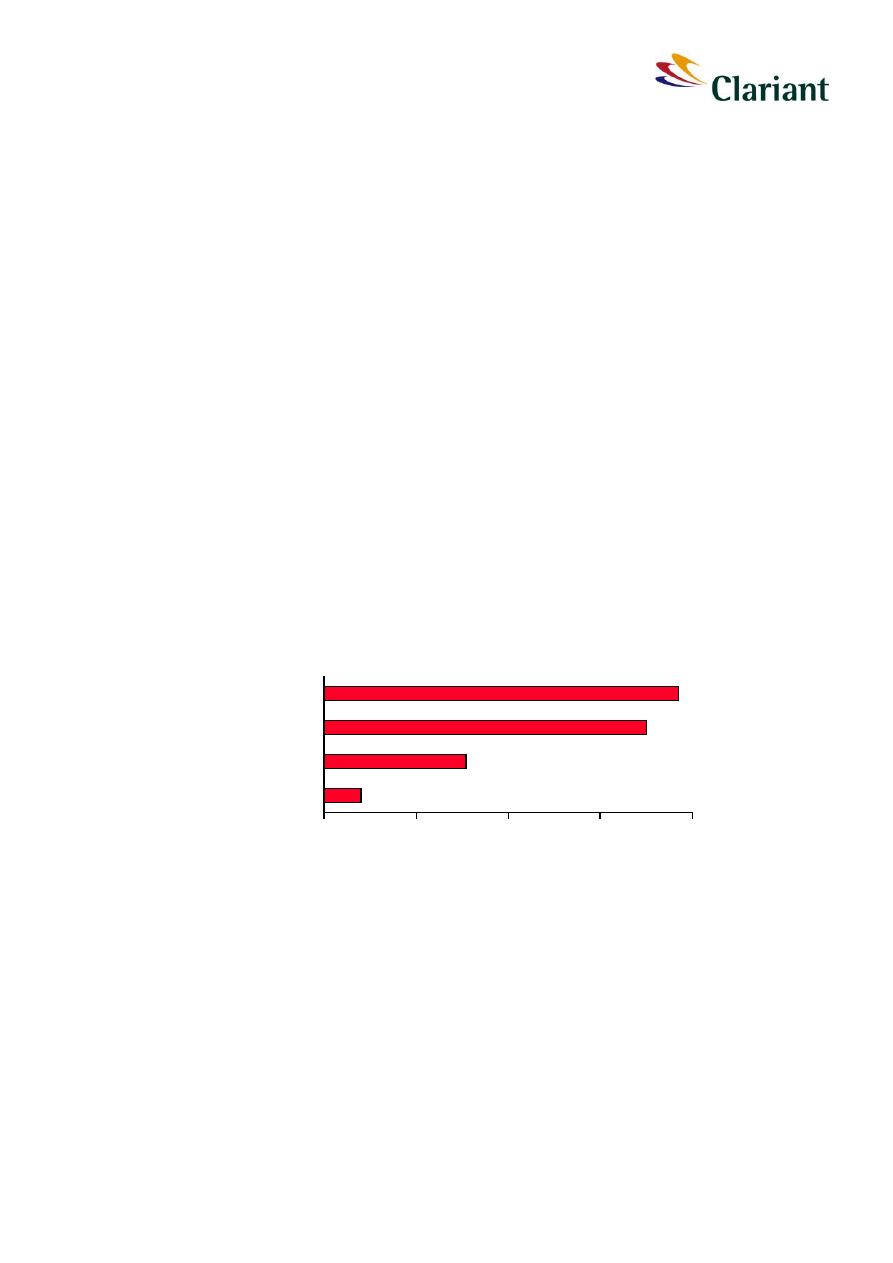
About 7 000 tons per year of red phosphorus are produced world wide. The most important
producers of red phosphorus are located in Europe, in Germany and Italy in particular. The
overall estimated capacity in the world is shown in Figure 1. The main market is Europe
followed by China and India.
Red Phosphorus is produced by thermal conversion of white phosphorus. The obtained red
phosphorus still contains up to about 100 mg/kg of white phosphorus. The upper limit given
in the product data sheets is 200 mg/kg white phosphorus. Red phosphorus is a red powder
which can be handled easily compared to the safety precautions necessary when handling
white phosphorus. Main applications are matches, aluminium phosphide and flame retar-
dants (see Figure 2).
0
1 000
2 000
3 000
4 000
Japan
India
China
Europe
red phosphorus volume (tons)
at least 6
producers
3 producers
Figure 1:
Global capacities for production of red phosphorus (total is 9 300 tons)
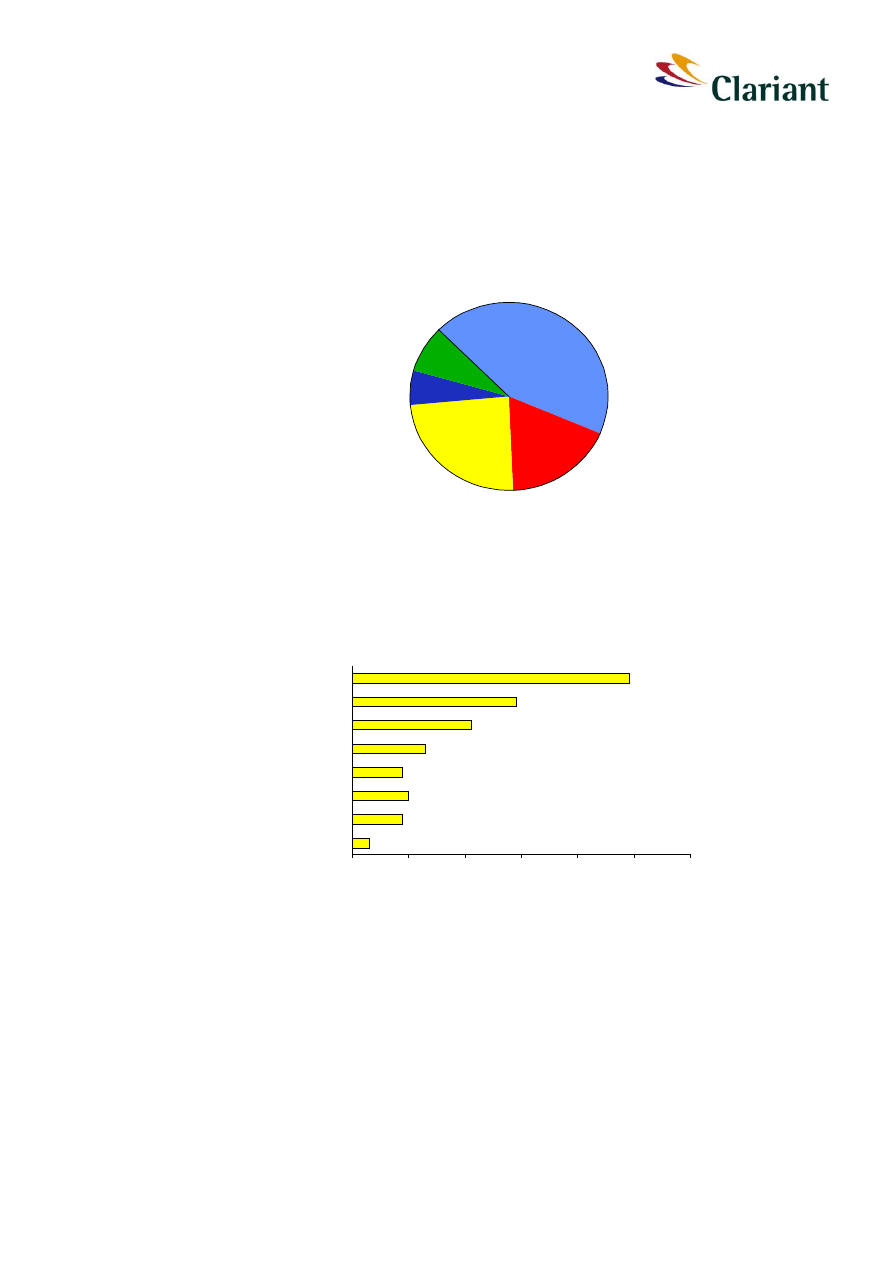
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
5
P-red-AquaChem-06.doc - 14.01.00
Matches
44%
Flame Retardants
18%
Aluminium phosphide
24%
Pyrotechnics
6%
others
8%
Figure 2:
Application areas of red phosphorus. Total global demand is about 7 150 tons.
0
500
1'000
1'500
2'000
2'500
3'000
Africa
South America
NAFTA
Japan
rest of Asia
India
China
Europe
R
e
gi
ons
Market volume (tonne)
Figure 3:
Red phosphorus market by key regions

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
6
P-red-AquaChem-06.doc - 14.01.00
The major distributor for red phosphorus for matches is located in Europe. Europe is also
the principal market for red phosphorus due to the production of aluminium phosphide and
the use as flame retardant in plastics. In Europe red phosphorus plays an important role as
a flame retardant for plastics, especially for polyamides in electronics, polyurethanes and
latex. Despite of its red colour it is used because in some cases it is the only material which
meets the high safety requirements for the end products combined with the high technical
requirements. In polyamides for electrical and electronic applications it is preferred because
of its high stability which enables the use of such plastic materials up to high voltages.
2.2
Red phosphorus: short process description
Red phosphorus is one of the allotropic forms of elemental phosphorus. It is largely amor-
phous and is considered a polymeric version of white phosphorus. Commercial red phospho-
rus is normally produced by heating the white phosphorus at a temperature range of 250 –
350 °C for 40 – 50 hours in a closed furnace (exclusion of oxygen) and at ambient pres-
sure.
After the polymerisation, the product is milled in presence of water then treated with an al-
kaline solution in order to remove traces of white phosphorus and finally filtered, washed
and dried. Although this special treatment is applied for removing white phosphorus in the
production process, some mg/kg of white phosphorus still remain in the final product.
Specifications of commercial red phosphorus are presented in annex 1. The specifications
state that the level of white phosphorus is < 200 mg/kg, but the present technology is
able to reduce the white phosphorus content to < 100 mg/kg.
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
7
P-red-AquaChem-06.doc - 14.01.00
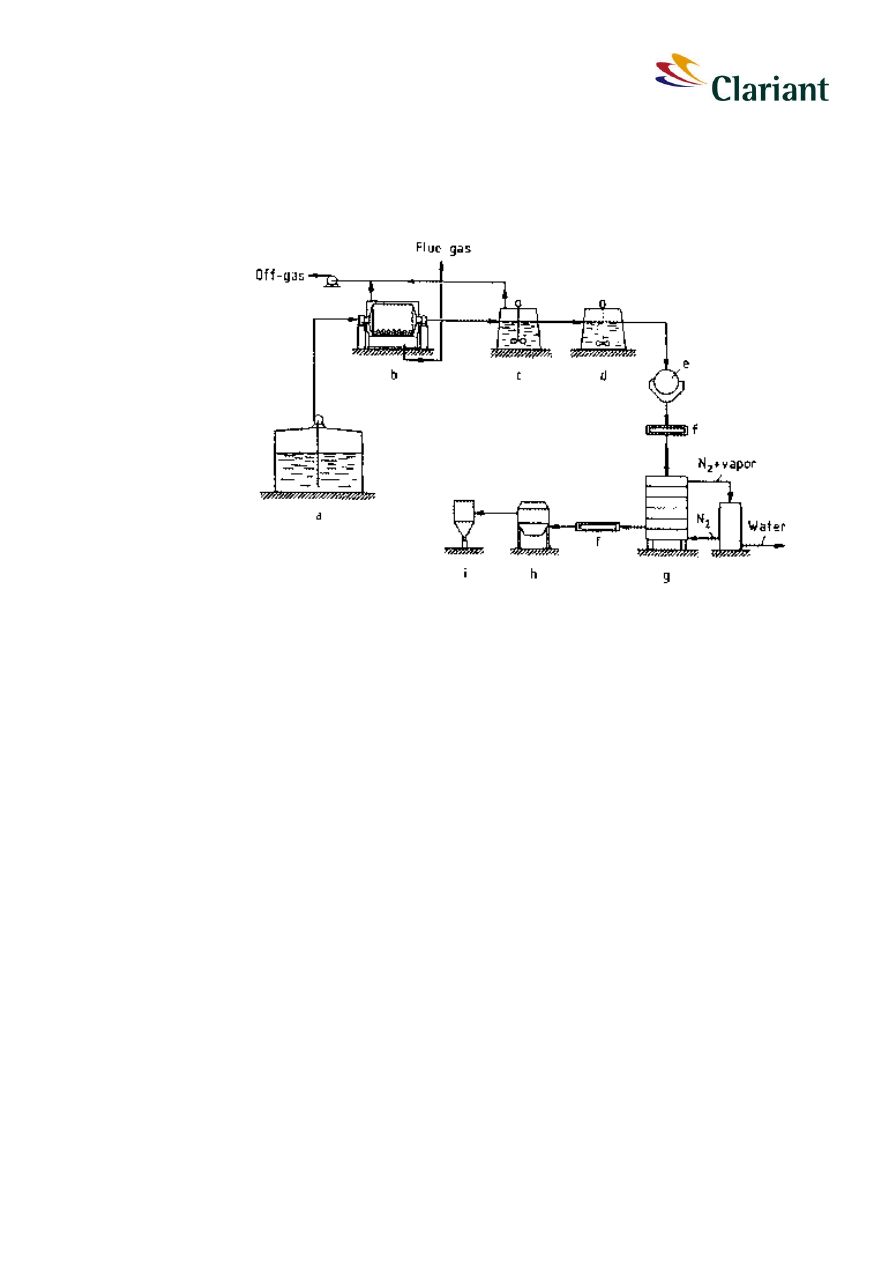
Figure 4:
Manufacturing process of red phosphorus
1
with a) phosphorus storage, b) reactor, c)
+ d) treatment of phosphorus-water mixture, e) filtration, f) conveyor, g) dryer, h) air
classification and sieving, i) predelivery storage
1
Ullmann’s Encyclopedia of Industrial Chemistry, Sixth Ed., 1999 – PHOSPHORUS, Wiley-VCH, Weinheim,
Germany

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
8
P-red-AquaChem-06.doc - 14.01.00
2.3
Characteristics of red phosphorus in comparison to white phosphorus
Table 1:
Comparison of white and red phosphorus
white phosphorus
red phosphorus
CAS-number
12185-10-3
7723-14-0
structure
contains discrete P
4
molecules
highly polymeric P
n
appearance
crystalline, waxy, translucent
amorphous or crystalline, opaque
melting point
44 °C
585 – 610 °C
vapour pressure
high
very low
density
1.83 g/cc
2.0 ... 2.4 g/cc
solubility:
in organic solvents:
in water:
yes
only ~3 mg / L
no
no (very slow hydrolysis)
toxicity
highly toxic
very low toxicity
heat of sublimation
13.4 k cal/mol
30.0 k cal/mol
chemiluminescence
yes
no
ignitability
-
spontaneous ignition in air at
room temperature
-
spontaneously ignites in chlorine
-
ignites only above 260 °C
-
heat necessary for ignition in
chlorine
smell
characteristic
no smell
reaction with aqueous
alkali
produces phosphine
none
The most important characteristics of red phosphorus in comparison to white phosphorus
are summarized in Table 1. These data show that the two products do have only little in
common, as a matter of fact they are far different in chemical structure, physico-chemical
properties, reactivity and toxicity.
The term red phosphorus is used for describing a variety of different amorphous forms of
the elemental phosphorus showing a range of colours from the orange to dark-violet. Such
differences in colour can be explained by differences in:
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
9
P-red-AquaChem-06.doc - 14.01.00
• particle size of the powder,
• molecular weight,
• impurities normally present on the red phosphorus surface
2
.
Although the red phosphorus is largely amorphous, X-ray diffraction, optical microscopy,
and differential thermal analysis (DTA) have established the existence of several crystalline
red varieties of pure elemental P in addition to the amorphous form. Normally the commer-
cial red phosphorus is amorphous and the crystalline form is present only to a limited extent
(< 10 % w) which is due to the ordered framework of different degrees of polymerisa-
tion
2
.
Red phosphorus has been described as a complex three-dimensional polymer in which each
P atom has a pyramidal arrangement of three bonds linking it to neighbouring P atoms as
shown in Figure 5.
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
P
=
Figure 5:
Structures of white and red phosphorus: tetrahedral structure of white phosphorus
on the left and a possible substructure of red phosphorus on the right with three di-
mensional view (bottom); the grey circles symbolize free valences where the chain
could continue or where terminal groups like –H or –OH or other heteroatoms (from
impurities) could be attached.
2 Corbridge, D.E.C.: Studies in inorganic Chemistry 10 – Phosphorus: an outline of its chemistry, biochemistry
and technology (Fourth edition). Elsevier 1990 pp. 56-65

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
10
P-red-AquaChem-06.doc - 14.01.00
It seems likely that all forms of red phosphorus are built from the pyramidal white phospho-
rus structure (Figure 5), and that the polymer growth is terminated by the occluded impuri-
ties such as halogen, oxygen or hydroxyl groups.
In conclusion, the amorphous red phosphorus probably consists of entirely random net-
works of P atoms terminated by oxygen or hydroxyl groups as depicted in Figure 5. This
assumption is confirmed by NMR spectra of the solid red phosphorus material. Due to the
fact that red phosphorus is a polymer, it is not a surprise that physico-chemical characteris-
tics, the reactivity and the stability are far different from white phosphorus: the white
phosphorus is crystalline, contains discrete P
4
molecules, has a melting point of about 44
°C, is very soluble in organic solvents like CS
2
and benzene, is very unstable and spontane-
ously ignites in presence of air. Whereas white phosphorus is a very toxic product, the red
phosphorus is not toxic (LD 50 oral rat is > 2000 mg/kg) as expected on the basis of the
polymeric structure.
2.4
Content of white phosphorus in red phosphorus
Even with state of the art technology it cannot be prevented that red phosphorus contains
traces of white phosphorus. This amount of white phosphorus is determined by extraction
with carbondisulfide and subsequent measuring by phosphorus-NMR spectroscopy in com-
parison with a standard sample of white phosphorus in toluene. The average content of
white phosphorus in commercial red phosphorus is normally about 50 – 100 mg/kg, even if
the upper limit given in the specifications is 200 mg/kg.

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
11
P-red-AquaChem-06.doc - 14.01.00
3
Solubility of white phosphorus in water and related reactions
White phosphorus is hardly soluble in water (about 3 mg/L) so that it can be stored and
transported under a protective aqueous layer. A comprehensive study on the literature of
the chemical behaviour of phosphorus in the environment has been carried out
3
. If white
phosphorus is exposed to both water and air under conditions in which it will not ignite, a
complex mixture of oxyacids of phosphorus and phosphine is slowly formed at room tem-
perature. The following chemical reactions occur in water (only qualitative description):
P
4
+ H
2
O + O
2
H
3
PO
2
+ H
3
PO
3
+ H
3
PO
4
+ PH
3
Measurements carried out by Clariant by P-NMR could not detect any phosphine in aqueous
extracts of white phosphorus (detection limit of 1 mg/L). As far as the white phosphorus it-
self is concerned it is not clear whether the solubility of 3 mg/L is due to completely dis-
solved phosphorus or partly suspended colloidal particles. Anyway, this may be the reason
for the extraordinary toxicity of white phosphorus to aquatic animals, because it might be
incorporated in elemental form. Maddock and Taylor4 claimed to have detected elemental
P4 in the organs of cod fish.
This is a fundamental difference to red phosphorus were no elemental phosphorus in water
can be observed (see chapter 4.2). Even the amount of white phosphorus detected in the
red phosphorus can not be extracted or dissolved in water.
3
SRI International Project LSU-4937-I “Environmental fate of white phosphorus/felt and red phosphorus/butyl
rubber military screening smokes: Phase I – Literature Review”
4
Maddock B.G. and D. Taylor, Water Research, Vol. 10, pp 289-294 Pergamon Press 1976

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
12
P-red-AquaChem-06.doc - 14.01.00
4
Solubility of red phosphorus in water and related reactions
4.1
Theoretical approach
Red phosphorus shows a totally different chemical behaviour than white phosphorus. Red
phosphorus is a polymeric allotropic modification of phosphorus. White phosphorus consists
of reactive P
4
– tetrahedra (molecular weight: 124 g/mol) whereas red phosphorus has a
polymeric structure of P
n
. Consequently, the reactivity of red phosphorus is much lower
than of white phosphorus. Yellow phosphorus has to be handled under water otherwise it
will start to burn spontaneously. White phosphorus has a wax like appearance whereas red
phosphorus is a red to violet coloured powder which can be handled in air. The vapour pres-
sure of white phosphorus at 25 °C is 0.05 mbar whereas red phosphorus has no detectable
vapour pressure at this temperature.
Due to these differences the reaction velocity of red phosphorus with water is much slower
compared to white phosphorus, but the main reaction products are also phosphorus-
containing acids (see Figure 6). If the total amount of white phosphorus contained in com-
mercial red phosphorus (upper limit 200 mg/kg) were to dissolve in water, the following
concentrations given in Table 2 would be achieved. One has to bear in mind that white
phosphorus also reacts with water so that these calculated amounts of white phosphorus
can only be achieved theoretically. Laboratory experiments revealed that the yellow phos-
phorus contained in red phosphorus is not readily extractable with water.
Table 2:
Theoretical concentrations of white phosphorus in water from a
dispersion of solid red phosphorus containing 200 mg/kg of white
phosphorus
red phosphorus loading white phosphorus
10 mg / L
2 µg / L
100 mg / L
20 µg / L
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
13
P-red-AquaChem-06.doc - 14.01.00
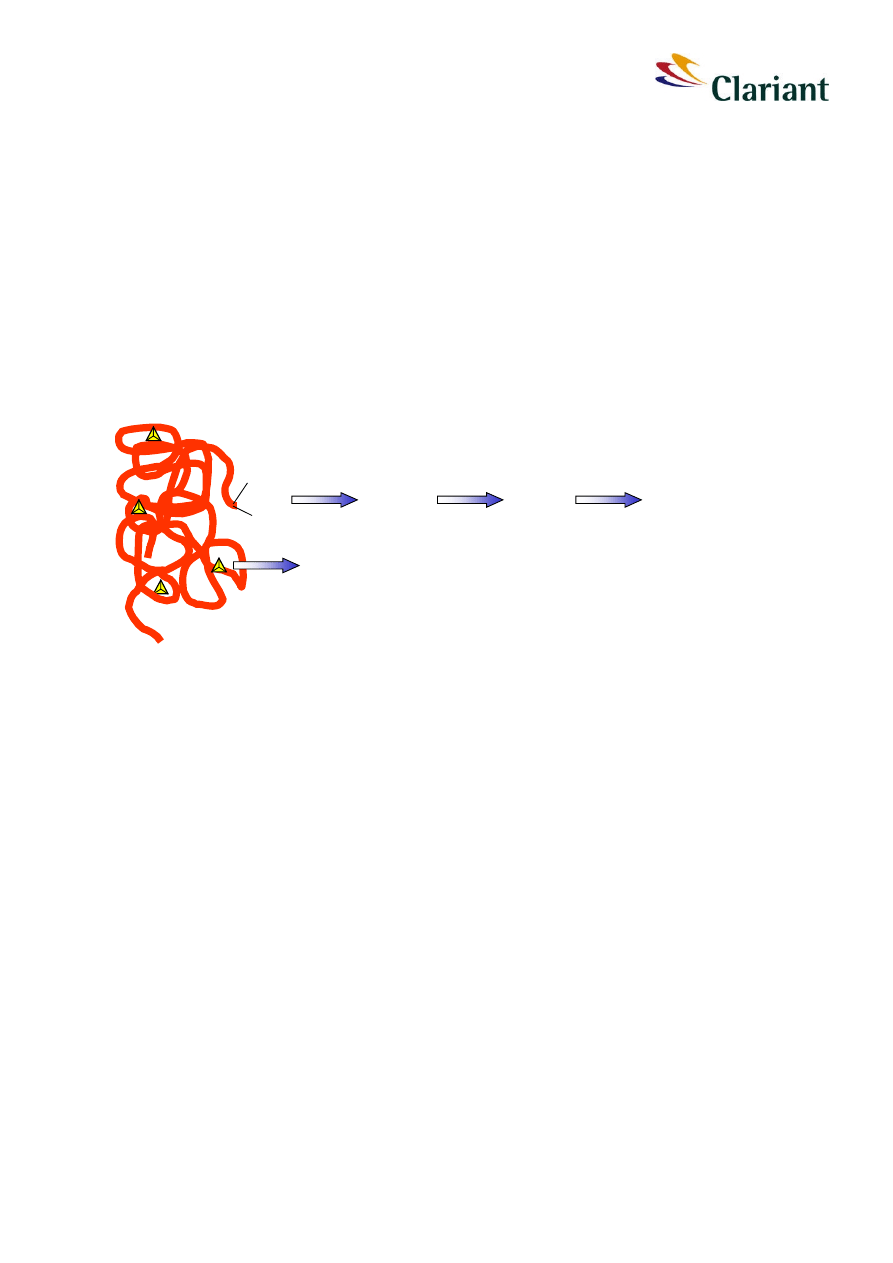
red phosphorus, amorphous polymer
yellow phosphorus (P
4
, max. 200 mg/kg)
no spontaneous
dissolution
OH
H
H
3
PO
2
+
X
H
3
PO
3
H
3
PO
4
1
2
3
4
X
Figure 6:
Schematic overview of reactions of red phosphorus in water. Annotations to the indi-
vidual reactions:
1
initial reaction is disproportionating or oxidation of phosphorus in the
polymer chain; this is the overall rate limiting step
2
the first products in aqueous solution are hypophosphorous acid and
at least one unknown compound X; phosphine (PH
3
) is also formed as
an intermediate in trace amounts
3 + 4
further oxidation via phosphorous acid to phosphoric acid
4.2
Experimental data
4.2.1 Phosphorus-containing acids
Red phosphorus reacts very slowly with water according to the reaction scheme presented
in Figure 6. The reaction products can be detected by NMR or ion chromatography. Clariant
carried out a short term (24 hours) and a long term (4 months) experiment with red phos-
phorus stirred in water (see Table 3 and Table 4). The amount of these phosphorus com-
pounds has been calculated as mg/L phosphorus for a straightforward comparison of
values. From these experimental results the following conclusions can be derived:
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
14
P-red-AquaChem-06.doc - 14.01.00
• The reactions rates of red phosphorus with water are very slow. The overall conversion
rate has been found to be always in linear correlation with the amount of the starting
material as well as the stirring time of red phosphorus in water.
• By taking in account the reasonable stirring time of 24 h only 0.2 % of the starting ma-
terial reacts with water.
• The reaction speed of red phosphorus in water is very slow and probably will never
reach a steady state because the resulting oxo-acids are very well soluble in water.
• We were not able to detect any elemental phosphorus in water
Table 3:
Reaction products after stirring 3 000 mg red phosphorus for 24 h in 1 liter of water
concentration
calc. as P [mg/L]
fraction
hypophosphite
1.0
18 %
phosphite
2.1
39 %
phosphate
1.4
26 %
unknown compounds
0.9
17 %
total phosphorus compounds
= fraction of nominal concentration
5.4
0.18 %
(of 3 000 mg/L)
100 %
Table 4:
Reaction products of 10 000 mg red phosphorus in 1 liter of water after 1 and 4
months
after 1 month
after 4 months
concentration
calc. as P [mg/L]
fraction
concentration
calc. as P [mg/L]
fraction
hypophosphite
19
19 %
50
19 %
phosphite
38
39 %
105
39 %
phosphate
34
35 %
98
36 %
unknown compounds
7
7 %
17
6 %
total phosphorus compounds
= fraction of nominal concentration
(10 000 mg/L)
98
0.98 %
100 %
270
2.7 %
100 %

BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
15
P-red-AquaChem-06.doc - 14.01.00
4.2.2 No release of white phosphorus from red phosphorus in water
Elemental phosphorus could not be detected in any of the aqueous red phosphorus suspen-
sions of the experiments in Table 3 and Table 4. The detection limit was 1 mg/L. To demon-
strate that the white phosphorus contained in the solid red phosphorus does not dissolve in
water (in contrast to pure white phosphorus which has a solubility of about 3 mg/L), the
following experiments were performed with a type red phosphorus containing the relatively
high concentration of 129 mg/kg white phosphorus:
a) 50 g of red phosphorus were extracted 3 times with 2.5 L of water for one hour. After
extraction the concentration of white phosphorus in the solid red phosphorus had only
slightly decreased to 122 mg/kg. This apparent reduction from the starting concentra-
tion is not significant, because it is within the range of the analytical error which
amounts to ± 5 mg/kg. Furthermore, no elemental phosphorus could be detected in the
water (limit of detection 1 mg/L).
b) 93 g of red phosphorus were extracted with 300 mL of water for three hours. The wa-
ter was subsequently extracted with carbon disulfide (CS
2
) and analysed for white
phosphorus – no phosphorus could be detected at a limit of detection of 0.1 mg/L.
In summary, no elemental white phosphorus could be extracted from red phosphorus with
water. Therefore, for instance an ecotoxicologic assessment of red phosphorus in the
aquatic environment cannot be based on its content of white phosphorus but should be
based on seperate studies with red phosphorus.
If traces of white phosphorus are released from the red phosphorus, they probably quickly
react to phosphorus containing acids – the same products that the red phosphorus itself
liberates. The source of these phosphorus containing acids be it white or red phosphorus
cannot be distinguished by chemical analysis, because the products themselves are identi-
cal and red phosphorus as a starting material is present in immense excess.
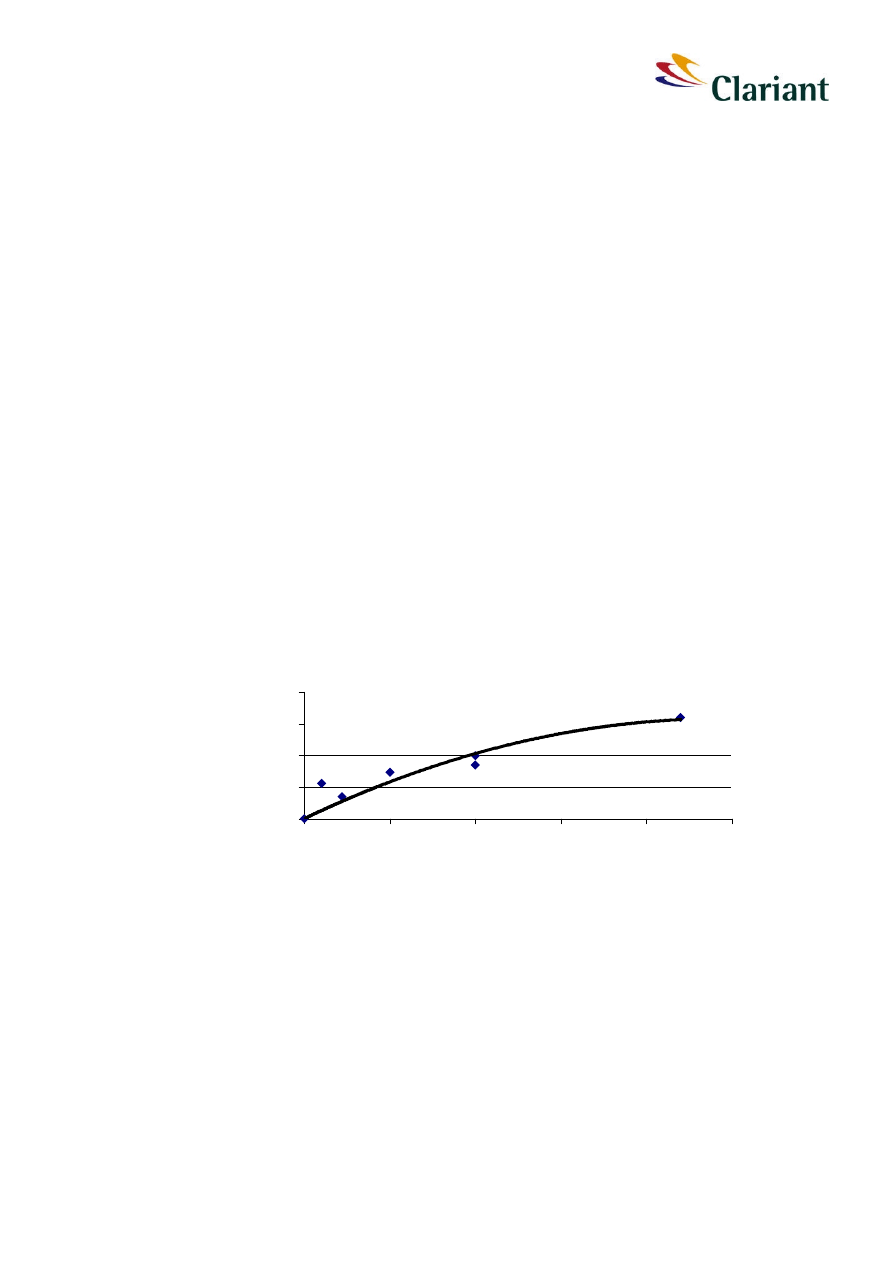
BL Flame Retardants
Walz R, Beard A
Chemical Behaviour of
Red Phosphorus in Water
16
P-red-AquaChem-06.doc - 14.01.00
4.3
Reaction rate of red phosphorus with water
Figure 7 indicates that the concentration of obtained hydrolysis products from red phospho-
rus steadily increases with the amount of dispersed red phosphorus in water. However, the
reaction of red phosphorus with water is extremely slow. The average amount of reaction
products from 100 mg/L after 24 h calculated as phosphorus is about 0.7 %. This fraction
rises very slowly up to a maximum of 3.7 % of the nominal concentration of solid red phos-
phorus in water after 700 hours. In another experiment the soluble reaction compounds in-
creased only up to 2.7 % after 2 880 hours (4 months). These data show that red
phosphorus does not dissolve as such in water which renders the concept of a maximum
solubility unapplicable. Instead, a continuous but slow series of reaction occurs leading to
phosphorus containing acids. The amount of products formed increases with the available
amount of red phosphorus and time.
Fraction of soluble P-compounds versus
amount of red P
(least squares 2nd order polynome)
0.0
0.5
1.0
1.5
2.0
0
50
100
150
200
250
red P, nominal concentration of solid material [mg/L]
total P in solution [mg/L ]
Figure 7:
Concentration of phosphorus compounds (calculated as total phosphorus) in aqueous
solution versus the amount of added solid red phosphorus
Wyszukiwarka
Podobne podstrony:
Determination of carbonyl compounds in water by derivatizati
Selective Functionalization of Amino Acids in Water
16 197 208 Material Behaviour of Powder Metall Tool Steels in Tensile
11 Fatigue behaviour of misaligned butt welded joints in the bottom flange
Hydrogen absorption behavior of beta titanium alloy in acid
AUTOMATED SECURITY HARDENING OF RED HAT ENTERPRISE LINUX V5 IN ACCORDANCE WITH DISA STANDARDS CSC Pa
Short term effect of biochar and compost on soil fertility and water status of a Dystric Cambisol in
Dance, Shield Modelling of sound ®elds in enclosed spaces with absorbent room surfaces
Proteomics of drug resistance in C glabrata
Estimating Temperatures in a Water
Microstructures and stability of retained austenite in TRIP steels
MMA Research Articles, Risk of cervical injuries in mixed martial arts
Development of financial markets in poland 1999
Antigone Analysis of Greek Ideals in the Play
Analysis of Police Corruption In Depth Analysis of the Pro
Low Temperature Differential Stirling Engines(Lots Of Good References In The End)Bushendorf
01 [ABSTRACT] Development of poplar coppices in Central and Eastern Europe
więcej podobnych podstron