
Carcinogenesis
vol.28 no.5 pp.1040–1045, 2007
doi:10.1093/carcin/bgl237
Advance Access publication December 13, 2006
Evaluation of the role of Finnish ataxia-telangiectasia mutations in hereditary
predisposition to breast cancer
Katri Pylka¨s
y
, Johanna Tommiska
1,
y
, Kirsi Syrja¨koski
4
,
Juha Kere
5,6
, Magtouf Gatei
7
, Nicola Waddell
7
, Minna
Allinen, Sanna-Maria Karppinen, Katrin Rapakko, Helena
Ka¨a¨ria¨inen
8
, Kristiina Aittoma¨ki
2
, Carl Blomqvist
3
, Aki
Mustonen, Kaija Holli
9
, Kum Kum Khanna
7
, Olli-Pekka
Kallioniemi
4,10
, Heli Nevanlinna
1
and Robert Winqvist
Department of Clinical Genetics, University of Oulu/Oulu University
Hospital, FIN-90029 OYS Oulu, Finland,
1
Department of Obstetrics and
Gynecology,
2
Department of Clinical Genetics,
3
Department of Oncology,
Helsinki University Central Hospital, Biomedicum Helsinki, FIN-00029 HUS
Finland,
4
Laboratory of Cancer Genetics, Institute of Medical Technology,
University of Tampere/Tampere University Hospital, Tampere, Finland,
5
Department of Medical Genetics, University of Helsinki, Biomedicum
Helsinki, Finland,
6
Department of Biosciences at Novum and Clinical
Research Centre, Karolinska Institutet, Huddinge, Sweden,
7
Signal
Transduction Laboratory, Queensland Institute for Medical Research, Herston,
Qld, Australia,
8
Department of Medical Genetics, University of Turku, Turku,
Finland,
9
Medical School, University of Tampere, and Palliative Unit,
Tampere University Hospital, Tampere, Finland
10
Present address: Medical Biotechnology Unit, VTT Technical Research
Centre of Finland and University of Turku, Turku, Finland
To whom correspondence should be addressed. Tel:
þ358 8 3153228;
Fax:
þ358 8 3153243;
Email: robert.winqvist@oulu.fi
Biallelic mutations in the ataxia-telangiectasia mutated (ATM)
gene result in ataxia-telangiectasia (A-T). Studies on A-T families
have shown that obligate female carriers have increased risk of
developing breast cancer. Here we have evaluated the role of
known Finnish ATM germ line mutations as possible breast can-
cer predisposing alleles outside A-T families by analyzing their
prevalence in large cohorts of familial and unselected breast can-
cer cases. Of seven different alterations, two were observed in the
studied breast cancer material. ATM 6903insA (causing protein
truncation) was seen in 3/541 familial and 5/1124 unselected cases,
but not among healthy population controls (0/1107). 7570G.C
(Ala2524Pro) occurred in 1/541 familial and 2/1124 unselected
cases compared with 1/1107 in controls. Additionally, 8734A.G
(Arg2912Gly) associated previously with breast cancer suscepti-
bility and suggested to be causative also for A-T was detected in 2/
541 of familial cases, but not in unselected cases (0/1124) or con-
trols (0/1107). In total, heterozygous ATM mutation carriers were
observed in 6/541 familial [P 5 0.006, odds ratio (OR) 12.4, 95%
confidence interval (CI) 1.5–103.3) and 7/1124 unselected cases
(P 5 0.07, OR 6.9, 95% CI 0.9–56.4), compared with 1/1107 in
controls, suggesting an apparent yet overall limited contribution
to predisposition to cancer. The current results also provided
evidence for founder effects in the geographical distribution of
these mutations. Interestingly, results from functional analysis
of the breast cancer-associated ATM mutations indicated that
cancer susceptibility is not restricted to mutations with domi-
nant-negative effect on kinase activity, displayed only by
7570G.C, whereas 8734A.G showed only a partial defect in
the phosphorylation of ATM substrates, and 6903insA seemed to
be a null allele.
Introduction
Familial breast cancer is a heterogeneous disorder. Apart from BRCA1
and BRCA2, the lack of convincing evidence of additional major
susceptibility genes suggests that the remaining cases are due to mu-
tations in several other genes, perhaps with lower disease penetrance
(1). ATM has long been considered a good candidate gene. It encodes
a protein which is a major activator of cellular responses to DNA
double-strand breaks through subsequent phosphorylation of central
players in the DNA damage response pathways, including BRCA1,
p53, Chk2 and NBS1 (2).
Biallelic ataxia-telangiectasia mutated (ATM) mutations result in
ataxia-telangiectasia (A-T), a recessive disorder characterized by pro-
gressive neurodegeneration, cell cycle checkpoint defects, radiosen-
sitivity and increased risk of cancer (3). Studies on A-T families have
suggested that obligate female carriers have an increased risk of breast
cancer (4,5). However, the role of ATM as a breast cancer suscepti-
bility gene outside the A-T families has been controversial, as many
of the case–control studies have failed to show an elevated frequency
of truncating ATM mutations in breast cancer patients. This has been
explained not only by the use of breast cancer cases unselected for
family history, which might be an inefficient way to detect ATM
mutations, but also by that only ATM mutations with specific func-
tional consequences predispose to cancer (6). It has been suggested
that dominant-negative mutations, missense changes in particular,
which give rise to stable kinase-inactive or non-phosphorylable pro-
teins are the ones mainly responsible for the increased cancer risk in
ATM carriers (7,8). Yet, two recent studies in A-T families did not
identify mutation-specific differences in cancer risk (9,10).
In order to search for possible cancer susceptibility alleles in ATM,
we recently performed a full mutation analysis of the coding regions
and splice sites of the gene in 121 Northern Finnish breast cancer fam-
ilies, previously evaluated also for the presence of Finnish A-T-related
ATM mutations (11–13). Altogether, the analysis of this geographi-
cally constrained cohort revealed only two different heterozygous
mutations, 7570G.C (in two individuals) and 6903insA (in one in-
dividual), both of which have been identified previously in A-T pa-
tients. This suggests that breast cancer susceptibility alterations in
ATM mainly are restricted to those reported in A-T. Some other stud-
ies on familial cases have also provided evidence that A-T-causing
mutations are breast cancer susceptibility alleles in the general pop-
ulation (14–16). These findings prompted us to perform a more com-
prehensive analysis on the impact of ATM mutations, originally
identified in Finnish A-T patients, in hereditary predisposition to
breast cancer.
A large new cohort of 541 BRCA1 and BRCA2 mutation-negative
breast cancer families were screened for the presence of the following
A-T mutations: IVS14
þ 3-4delAT (exon 14 skipped), IVS37 þ
9A.G (insertion Val, Ser, Stop), 6779-6780delTA (truncation),
6903insA (truncation), 7570G.C (marked previously as 7522G.C,
Ala2524Pro), 8710-8715delGAGACA (deletion of Glu and Thr) and
9139C.T (Arg3047Stop). The frequencies of the observed ATM al-
leles were compared with those of 1124 breast cancer patients un-
selected for family history together with 1107 unaffected population
controls. The obtained results indicate contribution of germ line ATM
aberrations in cancer predisposition also outside A-T families. In ad-
dition, we provide evidence for functional consequences of three
observed breast cancer-associated ATM mutations.
Materials and methods
Subjects
Index cases of 541 BRCA1 and BRCA2 mutation-negative families were
screened for known Finnish A-T-related mutations and for the additional
Abbreviations:
AI, allelic imbalance; A-T, ataxia-telangiectasia; ATM,
ataxia-telangiectasia mutated; CI, confidence interval; IR, ionizing radiation;
LCL, lymphoblast cell line; OR, odds ratio.
yJoint first authorship.
Ó The Author 2006. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oxfordjournals.org
1040
at Pomorska Akademia Medyczna on October 17, 2011
carcin.oxfordjournals.org
Downloaded from

ATM germ line mutation, 8734A.G, observed during this study. Inclusion
criteria for the families were as follows: (i) three or more affected in the family
(285 cases); (ii) two affected first-degree relatives (251 cases) or (iii) two
affected second-degree relatives (5 cases). The frequencies of the observed
mutations were compared with those of geographically matched 1124 un-
selected breast cancer cases and 1107 healthy controls. All patients provided
informed consent for obtaining pedigree data and DNA specimens. Approval
for the study was obtained from the Ethical Boards of the involved University
Hospital health care districts and the Finnish Ministry of Social Affairs and
Health.
Mutation detection
Screening was performed by conformation-sensitive gel electrophoresis and
minisequencing (17,18). All findings were confirmed by re-amplification of the
original DNA sample and direct sequencing. Conformation-sensitive gel elec-
trophoresis primers for exons 14, 37, 62 and 65 have been reported previously
(19). Sequences for exons 48, 49, 53 and for minisequencing are available upon
request.
Microsatellite marker analysis
D11S1819, D11S2179, D11S1778, D11S1294 and D11S1818 markers were
used to determine the haplotype of observed mutation alleles, and to study
possible allelic imbalance (AI) in the tumors of one 6903insA and two
7570G.C carriers. The polymerase chain reaction products were analyzed
with the Li-Cor IR
2
4200-S DNA Analysis system (Li-Cor, Lincoln, NE) using
an IRD800-labeled forward primer. Allele intensity ratios were quantified with
the Gene Profiler 4.05 analysis program (Scanalytics, Fairfax, VA). AI was
calculated by the formula AI 5 (T2
N1)/(T1 N2), where T1/2 represents
tumor and N1/2 the corresponding normal alleles. A value .1.67 or ,0.60 was
considered to indicate AI, meaning that the intensity of one allele had de-
creased .40%.
Cell cultures
Lymphoblast cell lines (LCLs) were established from one 7570G.C
(BR04108), one 8734A.G (BR0510) and two 6903insA carriers (BR0996,
BR0997). Two healthy (BR0409, C3ABR) and two affected non-carrier LCLs
(BR0197, BR0122), together with two A-T LCLs (AT1ABR, L3), were used as
reference. BR0409 was derived from the same family as BR0996 and BR0997.
AT1ABR was established in Brisbane, Australia. L3 is an LCL established
from a North African Jewish A-T patient and was obtained from Dr Yosef
Shiloh (Tel Aviv University, Tel Aviv, Israel). LCLs were grown in RPMI 1640
medium containing 20% fetal calf serum,
L
-glutamine and antibiotics.
ATM expression and kinase activity analysis
The effects of the mutations on ATM expression and kinase activity were
evaluated by western blot analysis. Cellular extracts were prepared by re-
suspending the cells in lysis buffer and incubating the mixture on ice for 30
min. Supernatants were collected after centrifugation at 14 000g for 15 min at
4
°C. ATM was immunoprecipitated with anti-ATM polyclonal antibody against
the N-terminus of protein (residues 250–522). Immunoprecipitates were re-
solved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and
western blotted with the same anti-ATM antibody. For assessment of in vivo
ATM kinase activity, 40 lg of extracts from mock or irradiated cell (4 Gy) was
analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and
immunoblotted with appropriate antibody. The phosphorylation of two ATM
substrates, p53 (Ser15) and Chk1 (Ser317), and the ATM autophosphorylation
site Ser1981, was assessed before and after exposure to ionizing radiation (IR).
Cell survival after IR
The cell survival was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-
nyltetrazolium bromide assay. Cells (10 000) of each LCL were plated in
triplicate into a 96-well plate in 100 ll media. Cell survival was assessed 4 days
after exposure to 0.5, 1, 2 and 3 Gy from a
137
Cs source, at a dose rate of ~1.1
Gy/min. For assessment of the number of viable cells, 100 ll of 3-(4,5-dime-
thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution, 1 mg/ml in phos-
phate-buffered saline, was added to each well and incubated at 37
°C for 4 h.
The cells were pelleted by centrifugation and re-suspended in 150 ll dimethyl
sulfoxide. Plates were incubated for 1 h at room temperature and measured at
570 nm. Cell survival fraction was calculated relative to the number of viable
cells in the non-irradiated culture at 96 h. Experiments were performed at least
twice on each LCL.
6903insA mRNA expression analysis
mRNA was isolated from 6903insA carrier LCLs with the FastTrack
Ò2.0 Kit
(Invitrogen, Carlsbad, CA) and cDNA was synthesized using the RevertAid
TM
First Strand cDNA Synthesis Kit (Fermentas, Hanover, MD). The presence of
6903insA transcript in the mRNA pool was evaluated by direct sequencing
with cDNA-specific primers: forward 5#-CCTGATGGAAAAGGAAATGG-3#
and reverse 5#-GCCACAAA CCCTCAGACATT-3#.
Statistical analysis
The differences in carrier frequencies were analyzed by Fisher’s exact test
(SPSS version 12.0 for Windows, SPSS). All P-values were two sided.
Results
ATM 6903insA and ATM 7570G.C are present in both familial and
unselected breast cancer cases
Of the seven Finnish A-T-related ATM mutations, 6903insA and
7570G.C (Ala2524Pro) were the only ones observed in the analyzed
breast cancer patients. ATM 6903insA was observed in the index
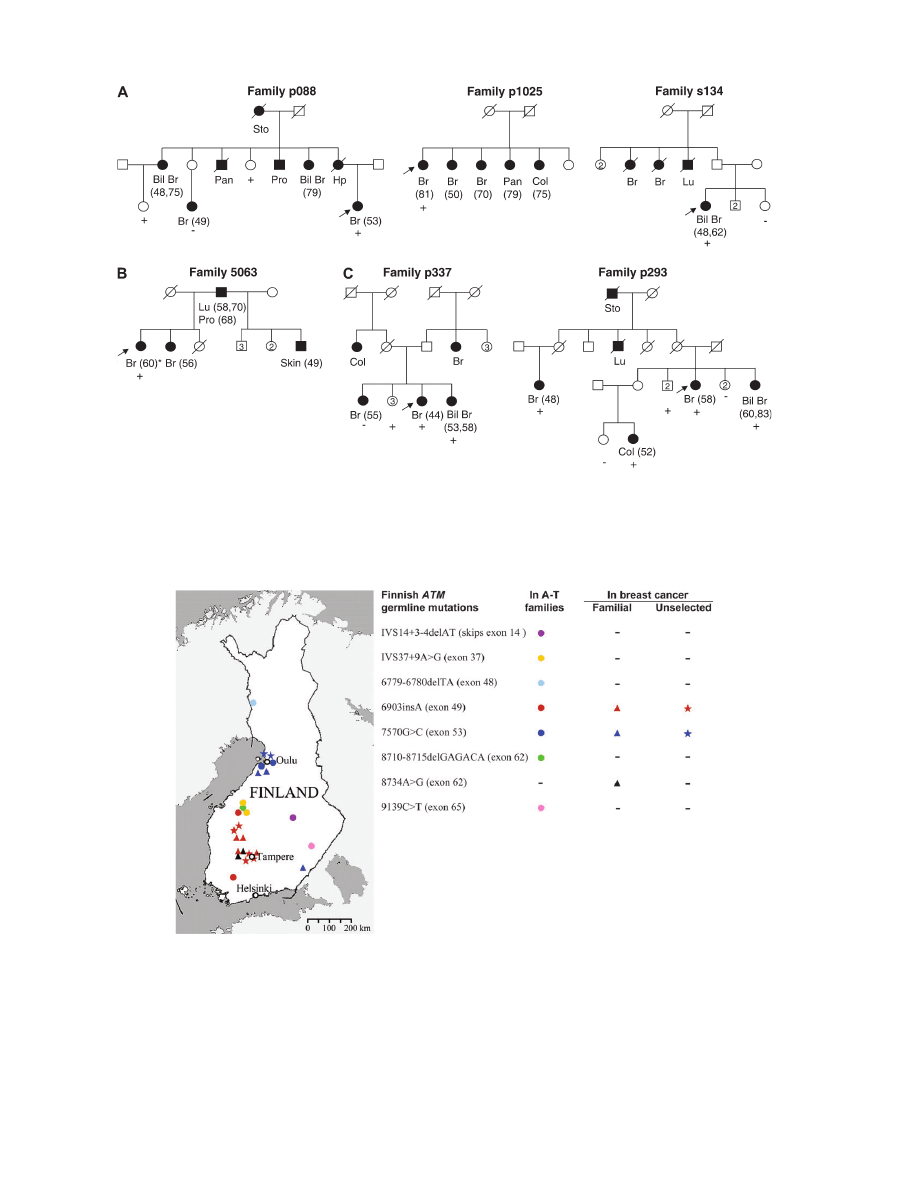
patients of three families (p088, p1025 and s134) (Figure 1A). The
study of additional family members in p088, however, showed in-
complete segregation with the disease. The prevalence of 6903insA
was also tested in 1124 unselected breast cancer cases and five car-
riers, diagnosed between the age 41 and 58, were observed. Cancer
registry inquiries revealed that at least one of the parents of four of
these cases had had cancer (lymphoma, uterine, bladder, esophageal
and stomach). All eight 6903insA carriers originated from the Tam-
pere region (Figure 2) and shared the same haplotype (data not
shown). 6903insA was not observed in the tested controls (0/1107).
ATM 7570G.C allele was observed in the index patient of family
5063 (Figure 1B). Interestingly, she had tested positive also for the
CHK2 1100delC alteration (20). Maternally the index originated from
central Finland and paternally from eastern Finland. The analysis of
1124 unselected breast cancer patients revealed two 7570G.C car-
riers, diagnosed at the age of 44 and 56 years, both originating from
the Oulu region (Figure 2). One 7570G.C carrier was identified also
among healthy controls. Because of the seemingly different geograph-
ical origins of some of the currently identified mutation carriers, a
microsatellite marker analysis was used to assess whether 7570G.C
derived from a common founder. Samples from eight different fam-
ilies, including two A-T and two breast cancer families identified in
previous studies (11,12), were analyzed. All carriers shared the same
haplotype (data not shown), thus confirming a common origin of the
mutation.
Breast cancer-associated ATM 8734A.G allele is observed in two
families
When screening for the A-T-related 8710-8715delGAGACA mutation
in exon 62 by conformation-sensitive gel electrophoresis, another al-
teration, 8734A.G (Arg2912Gly), was observed in the index patients
of two families (p337 and p293) (Figure 1C). 8734A.G has previ-
ously been associated with breast cancer susceptibility (15,21), and
has been suggested to be causative also for A-T (15). Consequently, it
was included in the study. Both 8734A.G positive families originated
from the Tampere region (Figure 2) and shared the same haplotype
(data not shown). However, the segregation of 8734A.G with cancer
was incomplete as several unaffected carriers occurred in both fam-
ilies, and in family p337, one mutation-negative breast cancer patient
was observed. 8734A.G was not observed among unselected cases or
in controls.
In the current study, ATM mutations, 6903insA, 7570G.C and
8734A.G, were observed altogether in 6/541 familial cases [P 5
0.006, odds ratio (OR) 12.4, 95% confidence interval (CI) 1.5–
103.3] and in 7/1124 unselected cases (P 5 0.07, OR 6.9, 95% CI
0.9–56.4), compared with only one 7570G.C carrier in 1107 healthy
controls (Table I). Thus far, in Finland, a total of 630 familial and
1209 breast cancer patients have been analyzed for known germ line
ATM mutations (current study, 12). The frequency of the observed
three ATM alleles is significantly higher in familial (9/630, P 5
0.0003, OR 18.9, 95% CI 2.4–149.7) and in unselected breast cancer
cases (7/1209, P 5 0.03, OR 7.6, 95% CI 0.9–61.9) compared with 1/
1307 in healthy controls.
Finnish ataxia-telangiectasia mutations and breast cancer
1041
at Pomorska Akademia Medyczna on October 17, 2011
carcin.oxfordjournals.org
Downloaded from
Loss of the wild-type allele is not implicated in the tumorigenesis of
ATM carriers
AI analysis was performed on available tumor samples of one 6903in-
sA and two 7570G.C carriers. None of the tumors showed loss of the
wild-type allele (data not shown).
The two missense mutations have no effect on protein stability
Heterozygous LCLs were used for the functional characterization
of the 7570G.C (BR04108), 8734A.G (BR0510) and 6903insA
(BR0996, BR0997) mutations. The amount of ATM from 7570G.C
and 8734A.G LCLs was comparable with that of the control subject
Fig. 2.
The geographical origin of Finnish A-T families (circle) (11), breast cancer families (triangle) and unselected breast cancer cases (star) displaying known
ATM germ line mutations. Minus (
) depicts absence of a certain mutation. Three previously reported mutation-positive breast cancer families (two with
7570G.C and one with 6903insA) are also included (12).
Fig. 1.
Families displaying (A) 6903insA, (B) 7570G.C and (C) 8734A.G mutations. Filled/open symbols indicate cancer/non-cancer status, respectively. Age
at diagnosis, when known, is shown after the cancer type (Bil Br, bilateral breast; Br, breast; Col, colorectal; Hp, hypopharyngeal; Lu, lung; Pan, pancreas; Pro,
prostate; Sto, stomach). Index cases are marked with an arrow, and subjects tested for a specific mutation ’
þ’ if positive and ’’ if negative. The subject marked
with an asterisk tested positive also for the CHK2 1100delC alteration (20).
K.Pylka¨s et al.
1042
at Pomorska Akademia Medyczna on October 17, 2011
carcin.oxfordjournals.org
Downloaded from
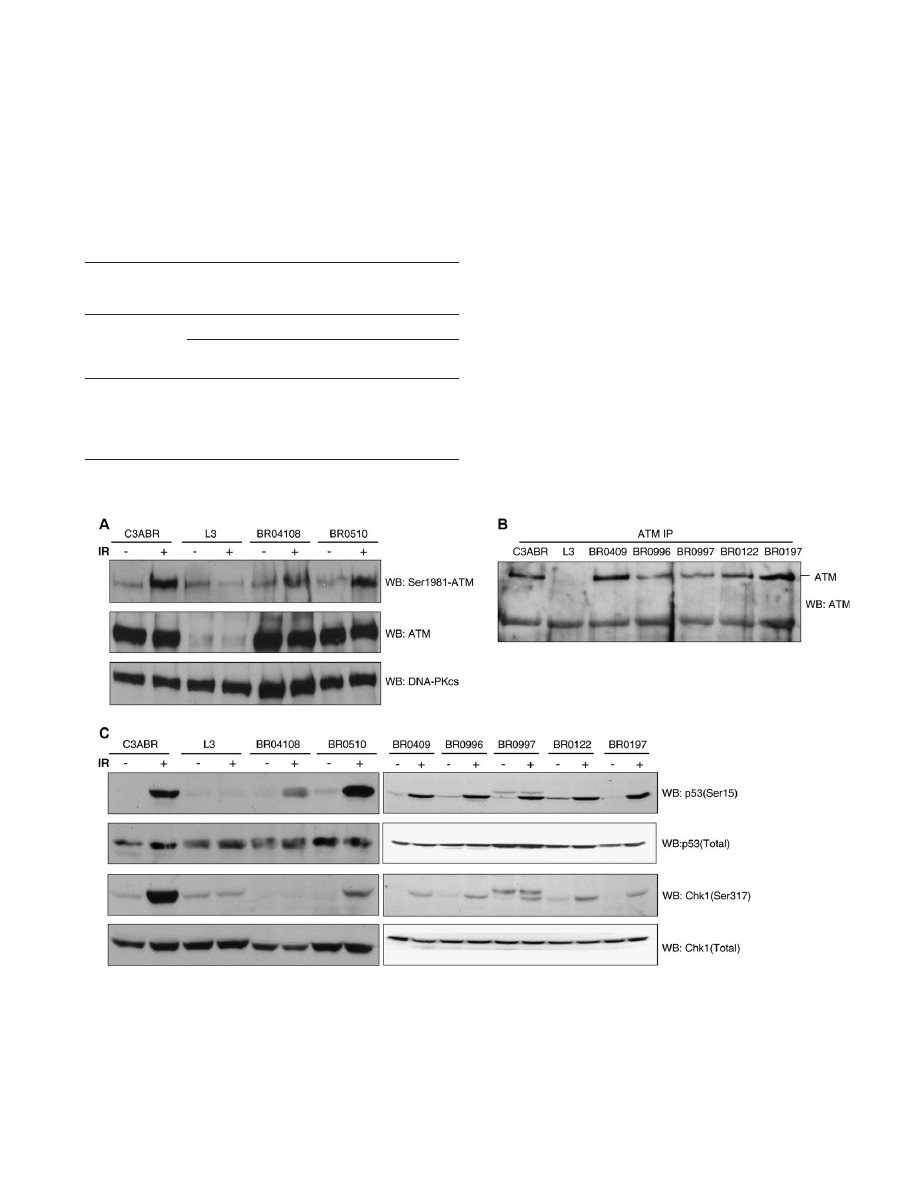
(C3ABR) homozygous for wild-type ATM (Figure 3A). For 6903in-
sA, leading to premature translation stop at codon 2372, no truncated
protein was observed and the level of full-length ATM expression was
reduced to half relative to the reference LCLs (Figure 3B). However,
sequencing analysis demonstrated that 6903insA transcripts were still
present in the tested mRNA pool.
7570G.C shows dominant-negative effect on ATM kinase activity
Possible downstream effects of the observed mutations were evalu-
ated by assessing the phosphorylation of two ATM substrates, p53
(Ser15) and Chk1 (Ser317), and the ATM autophosphorylation site
Ser1981 for the missense mutations 7570G.C and 8734A.G, before
and after exposure to IR (Figure 3A and C). In extracts from the
C3ABR control cell line, ATM was activated rapidly, as judged by
enhanced phosphorylation of p53 on Ser15 and Chk1 on Ser317. In
contrast, phosphorylation was barely detectable in the ATM non-ex-
pressing L3 cell line. DNA damage-induced phosphorylation of p53
and Chk1 was also dramatically lower in the 7570G.C carrier LCL
(BR04108), whereas the 8734A.G carrier LCL (BR0510) was de-
fective only in Chk1 phosphorylation (Figure 3C). For 6903insA
LCLs (BR0996, BR0997), no significant difference in the phosphor-
ylation was observed when compared with controls (Figure 3C).
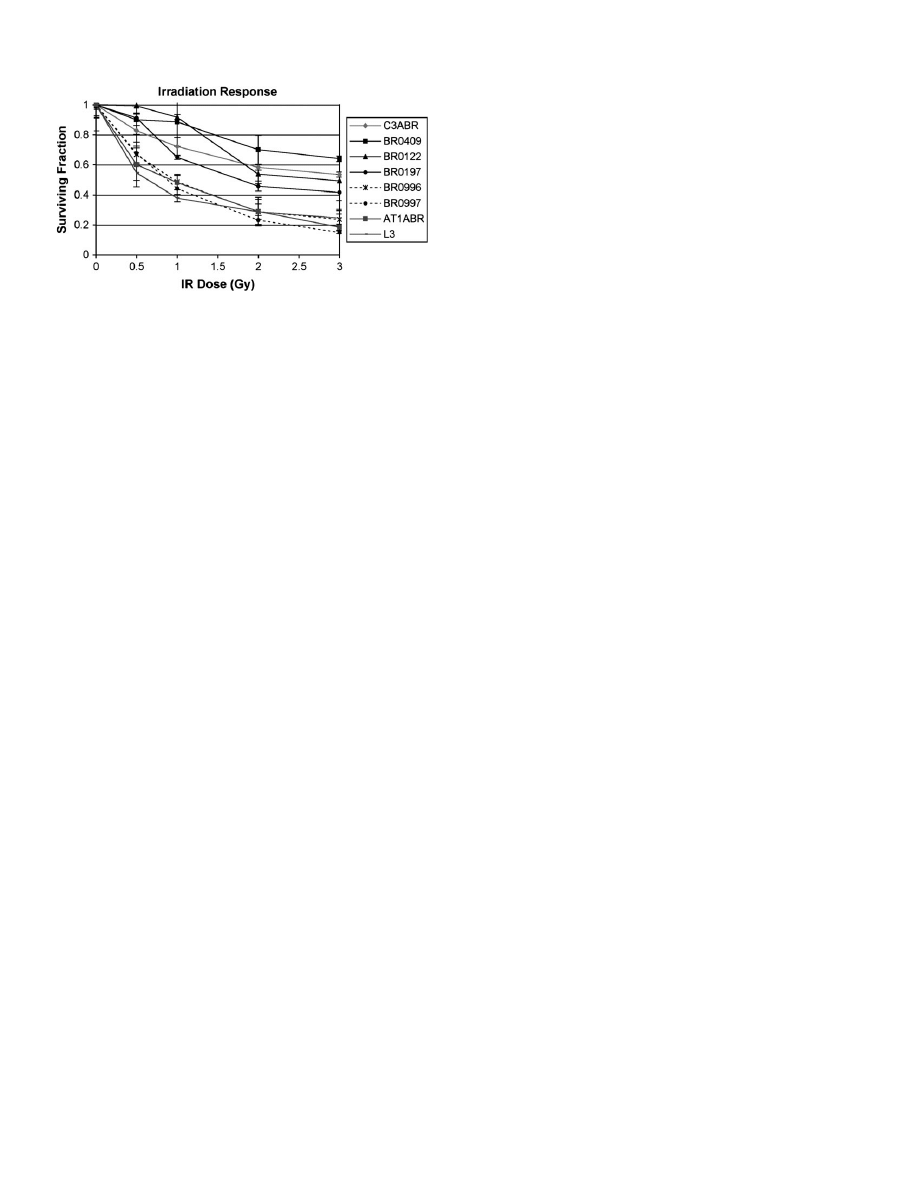
6903insA carrier LCLs show radiosensitivity
Since deficiency in ATM leads to increase in cellular sensitivity to IR,
the possible effects of 7570G.C, 8734A.G and 6903insA on radio-
sensitivity were evaluated. Two LCLs (L3 and AT1ABR) from A-T
patients served as positive controls. As expected, the A-T cell lines
displayed extreme sensitivity to IR when compared with healthy con-
trol LCLs (C3ABR, BR0409). The survival of the 7570G.C carrier
and 8734A.G carrier LCL was of the same order as the controls (data
not shown), whereas the survival of the two 6903insA heterozygous
cell lines (BR0996 and BR0997) was indistinguishable from that of
A-T cell lines at most dose points (Figure 4). Affected non-carrier
LCLs (BR0197, BR0122) showed radiosensitivity comparable with
the healthy control cell lines.
Table I.
Heterozygous ATM germ line mutations in Finnish breast cancer
families, unselected breast cancer cases and controls, observed in the current
study
ATM mutation
Carrier frequency (%)
Familial
cases
Unselected
cases
Controls
6903insA
a
0.6% (3/541)
0.4% (5/1124)
(0/1107)
7570G.C
a
0.2% (1/541)
0.2% (2/1124)
0.1% (1/1107)
8734A.G
0.4% (2/541)
(0/1124)
(0/1107)
Altogether
1.1% (6/541),
P 5 0.006
0.6% (7/1124),
P 5 0.07
0.1% (1/1107)
a
Observed in A-T patients.
Fig. 3.
ATM expression and kinase activity of mutation heterozygous LCLs. (A) ATM expression and Ser1981 phosphorylation in 7570G.C (BR04108) and
8734A.G (BR0510) carrier LCLs before (
) and after (þ) exposure to IR. Both cell lines show normal ATM protein levels, but only BR04108 exhibits defective
IR-induced ATM autophosphorylation. DNA-PKcs 5 control for protein loading. (B) Reduced expression of ATM in 6903insA carrier LCLs (BR0996 and
BR0997). A band of ~200 kDa precipitating non-specifically with the antibody has been used as control for protein loading. (C) In vivo analysis of ATM kinase
activity with p53 and Chk1 as substrates, before (
) and after (þ) exposure to IR. Kinase activity is shown by western blotting (WB) with anti-p53 Ser15 and anti-
Chk1 Ser317, and with additional anti-p53 and anti-Chk1 antibodies that detects the total pool, both phosphorylated and unphosphorylated, of these proteins.
Irradiation of BR04108 (7570G.C) cells results in grossly reduced phosphorylation of both p53 and Chk1. BR0510 (8734A.G) cells, on the other hand, show
only defective Chk1 phosphorylation. The origin of the supplementary bands in BR0997 is unknown. LCLs used are the following: BR04108 5 7570G.C carrier,
BR0510 5 8734A.G carrier, BR0996 and BR0997 5 6903insA carriers, BR0197 and BR0122 5 affected non-carrier LCLs, BR0409 and C3ABR 5 healthy
non-carrier cell lines, L3 5 A-T LCL.
Finnish ataxia-telangiectasia mutations and breast cancer
1043
at Pomorska Akademia Medyczna on October 17, 2011
carcin.oxfordjournals.org
Downloaded from
Discussion
We have assessed the relevance of seven ATM mutations, originally
identified in Finnish A-T patients, in hereditary predisposition to
breast cancer. The results suggest that two of these mutations, 6903in-
sA and 7570G.C, are breast cancer susceptibility alleles also outside
A-T families. The other five known alleles occurring in A-T were not
observed, which might be due to their low frequency. Yet, it is also
possible that some of these alleles do not predispose to breast cancer,
as it has been suggested that the risk might also depend on the location
of truncating mutations (9). Besides the 6903insA and 7570G.C
alleles, another ATM mutation, 8734A.G, was observed in two fam-
ilies. It has previously been associated with breast cancer susceptibil-
ity (15,21), and has been suggested to be causative also for A-T (15).
In Finland, 6903insA, 7570G.C and 8734A.G have been ob-
served altogether in 9/630 breast cancer families (P 5 0.0003, OR
18.9, 95% CI 2.4–149.7) (current study, 12). The study of additional
family members, however, showed incomplete segregation, as both
unaffected mutation carriers and mutation-negative breast cancer pa-
tients were observed. In addition, 6903insA and 7570G.C have also
been observed in breast cancer patients without known familial back-
ground of the disease (7/1209) (P 5 0.03, OR 7.6, 95% CI 0.9–61.9),
and 7570G.C also in one healthy control further suggesting incom-
plete penetrance for these mutations. Consequently, the breast cancer
risk associated with the observed ATM mutations is likely to depend
on environmental factors and/or susceptibility alleles in other genes,
as suggested by the polygenic model for breast cancer susceptibility
(1). This is consistent with the recent study, which found an ~2-fold
increase in risk of breast cancer associated with ATM mutations caus-
ing A-T. This risk appears to be similar to that of low-penetrance
susceptibility allele CHK2 1100delC (16). Overall, the observed
ATM mutations seem to explain only a small fraction of hereditary
susceptibility to breast cancer in Finland, as they have been observed
in 1.4% of the familial and 0.6% of the unselected cases studied so far.
The relatively low frequency of these alleles is not, however, surpris-
ing when considering the fact that at least two of them are also
pathogenic for A-T, thereby limiting their population frequency. Nev-
ertheless, as the observed alleles show geographical clustering, their
contribution to familial breast cancer in certain regions might be
significant. In particular, the 6903insA and 8734A.G mutations clus-
ter to the area of Tampere, and together, their frequency in the breast
cancer families studied from this region is 3.0% (5/168). In contrast,
7570G.C mainly concentrates to the Oulu region.
Previous studies have shown that loss of heterozygosity is not in-
volved in development of breast cancer in ATM carriers (22,23). Cor-
respondingly, none of the tumors tested showed loss of the wild-type
allele. Instead of the complete loss of normal protein, it has been
suggested that mutations with dominant-negative effect are the ones
mainly responsible for the increased risk of breast cancer in ATM
carriers. Subsequently, the functional consequences of all three ob-
served breast cancer-associated ATM mutations were investigated.
Analysis of the 7570G.C heterozygous cell line showed that sub-
stitution of the evolutionarily conserved (for instance, Mus musculus,
Xenopus laevis and Tel1p protein of Schizosaccharomyces pombe)
Ala2524 residue with proline in the FRAP/ATM/TRRAP (FAT) do-
main leads to a stable protein with defective kinase activity. Although
FAT contains no catalytic sequences, it occurs only in combination
with FRAP/ATM/TRRAP C-terminal (FATC), and it has been sug-
gested that these domains fold together in a configuration that ensures
proper function of the interposed kinase (24). Failure in correct fold-
ing could inactivate kinase functions, which could explain the patho-
genicity of 7570G.C. The defective phosphorylation of Ser1981, and
the two ATM downstream targets p53 Ser15 and Chk1 Ser317, seems
to be related to the dominant-negative effect of 7570G.C. This effect
has been shown also for another A-T-related and breast cancer-asso-
ciated mutation, 7271T.G (Val2424Gly), located in the FAT domain
(14,25). However, whereas the A-T patients homozygous for
7271T.G have been reported to have only mild clinical symptoms
(25,26), no difference in the disease phenotype of the two Finnish A-T
patients, one being compound heterozygote and the other homozy-
gous for 7570G.C, was observed (12).
The other observed A-T mutation, 6903insA, causes a frameshift.
No truncated protein was present in the carrier LCLs and the total
amount of endogenous ATM was reduced to about half. This seems
sufficient for normal function of the ATM checkpoint signaling path-
way, but not to ensure normal level of cell survival after IR-induced
damage. This differential impact on cellular radiosensitivity has been
reported previously by over-expression of ATM fragment containing
the leucine zipper domain that can act in a dominant-negative manner
to influence cell survival, but not p53 stabilization and cell cycle
checkpoints (27). If a truncated protein was expressed, although below
the level of the used detection method, it would contain the leucine
zipper and could potentially act in a dominant-negative way to influ-
ence cell survival. Alternatively, different biological endpoints and
functions could have different threshold requirements for ATM, which
also has been reported previously (28,29). Thus, although one cellular
pathway that might promote tumorigenesis is altered, others may func-
tion apparently normally. Accordingly, instead of the dominant-nega-
tive effect, haploinsufficiency might be a more plausible explanation
for the cancer susceptibility associated with 6903insA. The geograph-
ically confined high frequency among breast cancer patients and ab-
sence among healthy controls strongly suggest that 6903insA mutation
is associated with increased risk of developing cancer. Consequently,
breast cancer susceptibility is not restricted to ATM mutations with
dominant-negative effect on the kinase activity. This view is supported
by the results from two recent studies of A-T families, showing no
mutation-specific differences in cancer risk (9,10).
Besides the two A-T-related alleles, another potentially pathogenic
ATM mutation, 8734A.G, was observed. 8734A.G leads to
Arg2912Gly substitution in the kinase domain, altering a highly con-
served residue between different species and also in most other mem-
bers of phosphoinositide 3-kinase family kinases. However, the LCL
heterozygous for 8734A.G showed no defects in the phosphorylation
of ATM Ser1981 or p53 Ser15, whereas faulty Chk1 Ser317 phos-
phorylation was observed. Thus, Arg2912Gly substitution does not
impair the phosphorylation of all ATM substrates, and the cancer
predisposing effect of this mutation is not a dominant-negative one.
Nevertheless, Arg2912Gly may impair some other protein–protein
interactions required for optimal ATM kinase activity. Interestingly,
it has been shown that upon irradiation, the phosphorylation of Chk1
Ser317 by ATM is also dependent on NBS1 (30), and it has been
suggested that NBS1 assists ATM in targeting some of its substrates.
Consistent with this, it has been reported that phosphorylation of p53
by ATM occurs through an NBS1-independent mechanism (30,31).
Fig. 4.
Increased radiosensitivity of heterozygous ATM 6903insA LCLs. The
viable cells were counted by 3-(4,5-dimethylthiazol-2-yl)-2,5-
diphenyltetrazolium bromide assay 96 h after exposure of cells to indicated
dose of gamma radiation. Cell survival fraction was calculated relative to
number of viable cells in the non-irradiated culture at 96 h. Two different
A-T LCLs (AT1ABR and L3) were used as positive control for radiosensitivity.
BR0996 and BR0997 5 6903insA carrier LCLs, BR0197 and BR0122 5
affected non-carrier cell lines, BR0409 and C3ABR 5 healthy control LCLs.
K.Pylka¨s et al.
1044
at Pomorska Akademia Medyczna on October 17, 2011
carcin.oxfordjournals.org
Downloaded from

Even though the role of 8734A.G as a breast cancer susceptibility
allele has been reported previously (15,21), and is further supported
by the present results, so far it has not been reported in A-T cases.
Based on the current functional evidence, there might be a simple
explanation for this: although 8734A.G appears to predispose to
cancer, it may not be pathogenic enough to result in A-T clinical
phenotype when paired with itself or other mutant ATM alleles.
In conclusion, current results support the association of two A-T-
related ATM mutations, 6903insA and 7570G.C, in addition to
8734A.G, with breast cancer susceptibility. The results also provide
evidence for founder effects in the geographical distribution of these
alleles. The clustering of 6903insA and 8734A.G to the Tampere
region seems particularly strong, and together, these mutations re-
gionally contribute 3% of the studied familial breast cancer cases.
Of the observed changes, 7570G.C and 8734A.G lead to amino
acid substitutions, but only 7570G.C showed dominant-negative ef-
fect on kinase activity. For ATM 6903insA carriers, haploinsufficiency
might be a more plausible explanation for the predisposition to cancer.
Consequently, the results indicate that the ATM gene-dosage effect is
sufficient to exert a cellular phenotype that promotes tumorigenesis.
Acknowledgements
We thank Drs Guillermo Blanco, Ulla Puistola, Jaakko Ignatius and Hanna-
leena Eerola, and nurses Outi Kajula and Minna Merikivi for their help in
patient contacts. We thank Drs Anne-Lise Børresen-Dale and Jiri Bartek for
helpful discussions and Dr Veli Isomaa for support in protein analysis. The
technical assistance of Ms Arja Tapio, Ms Kati Outila, Ms Kati Rouhento and
Ms Sirpa Stick is greatly appreciated. This study was supported by the Acad-
emy of Finland, University of Oulu, Oulu University Hospital, Finnish Cancer
Society, Cancer Foundation of Northern Finland, Nordic Cancer Union, Maud
Kuistila Memorial Foundation, Clinical Research Fund of Helsinki University
Central Hospital and Sigrid Juselius Foundation. In particular, we thank all
patients participating in this study.
Conflict of Interest Statement: None declared.
References
1. Pharoah,P.D.P. et al. (2002) Polygenic susceptibility to breast cancer and
implications for prevention. Nat. Genet., 31, 33–36.
2. Shiloh,Y. (2003) ATM and related protein kinases: safeguarding genome
integrity. Nat. Rev. Cancer, 3, 155–168.
3. Lavin,M.F. et al. (1997) The genetic defect in ataxia-telangiectasia. Annu.
Rev. Immunol., 15, 177–202.
4. Swift,M. et al. (1987) Breast and other cancers in families with ataxia-
telangiectasia. N. Engl. J. Med., 316, 1289–1294.
5. Olsen,J.H. et al. (2001) Cancer in patients with ataxia-telangiectasia and in
their relatives in the nordic countries. J. Natl Cancer Inst., 93, 121–127.
6. Khanna,K.K. et al. (2004) ATM and genome maintenance: defining its role
in breast cancer susceptibility. J. Mammary Gland Biol. Neoplasia, 9,
247–262.
7. Gatti,R.A. et al. (1999) Cancer risk in ATM heterozygotes: a model of
phenotypic and mechanistic differences between missense and truncating
mutations. Mol. Genet. Metab., 68, 419–423.
8. Bakkenist,C.J. et al. (2003) DNA damage activates ATM through intermo-
lecular autophosphorylation and dimer dissociation. Nature, 421, 499–506.
9. Cavaciuti,E. et al. (2005) Cancer risk according to type and location of
ATM mutation in ataxia-telangiectasia families. Genes Chromosomes Can-
cer, 42, 1–9.
10. Thompson,D. et al. (2005) Cancer risks and mortality in heterozygous
ATM mutation carriers. J. Natl Cancer Inst., 97, 813–822.
11. Laake,K. et al. (2000) Characterization of ATM mutations in 41 nordic
families with ataxia telangiectasia. Hum. Mutat., 16, 232–246.
12. Allinen,M. et al. (2002) ATM mutations in Finnish breast cancer patients.
J. Med. Genet., 39, 192–196.
13. Heikkinen,K. et al. (2005) Association of common ATM polymorphism
with bilateral breast cancer. Int. J. Cancer, 116, 69–72.
14. Chenevix-Trench,G. et al. (2002) Dominant negative ATM mutations in
breast cancer families. J. Natl Cancer Inst., 94, 205–215.
15. Thorstenson,Y.R. et al. (2003) Contributions of ATM mutations to familial
breast and ovarian cancer. Cancer Res., 63, 3325–3333.
16. Renwick,A. et al. (2006) ATM mutations that cause ataxia-telangiectasia
are breast cancer susceptibility alleles. Nat. Genet., 38, 873–875.
17. Ko¨rkko¨,J. et al. (1998) Conformation sensitive gel electrophoresis for sim-
ple and accurate detection of mutations: comparison with denaturing gra-
dient gel electrophoresis and nucleotide sequencing. Proc. Natl Acad. Sci.,
95
, 1681–1685.
18. Syva¨nen,A.C. et al. (1993) Identification of individuals by analysis of
biallelic DNA markers, using PCR and solid-phase minisequencing. Am.
J. Hum. Genet., 52, 46–59.
19. Shayeghi,M. et al. (1998) Heterozygosity for mutations in the ataxia tel-
angiectasia gene is not a major cause of radiotherapy complications in
breast cancer patients. Br. J. Cancer, 78, 922–927.
20. Vahteristo,P. et al. (2002) A CHEK2 genetic variant contributing to a sub-
stantial fraction of familial breast cancer. Am. J. Hum. Genet., 71, 432–438.
21. Teraoka,S.N. et al. (2001) Increased frequency of ATM mutations in breast
carcinoma patients with early onset disease and positive family history.
Cancer, 92, 479–487.
22. Laake,K. et al. (1997) Loss of heterozygosity at 11q23.1 in breast carcin-
omas: indication for involvement of a gene distal and close to ATM. Genes
Chromosomes Cancer, 18, 175–180.
23. Broeks,A. et al. (2000) ATM-heterozygous germ line mutations contribute
to breast cancer-susceptibility. Am. J. Hum. Genet., 66, 494–500.
24. Bosotti,R. et al. (2000) FAT: a novel domain in PIK-related kinases. Trends
Biochem. Sci., 25, 225–227.
25. Stankovic,T. et al. (1998) ATM mutations and phenotypes in ataxia-
telangiectasia families in the British Isles: expression of mutant ATM
and the risk of leukemia, lymphoma, and breast cancer. Am. J. Hum. Genet.,
62
, 334–345.
26. Do¨rk,T. et al. (2001) Spectrum of ATM gene mutations in a hospital-based
series of unselected breast cancer patients. Cancer Res., 61, 7608–7615.
27. Morgan,S.E. et al. (1997) Fragments of ATM which have dominant-
negative or complementing activity. Mol. Cell Biol., 17, 2020–2029.
28. Delia,D. et al. (2000) ATM protein and p53-serine 15 phosphorylation in
ataxia-telangiectasia (AT) patients and at heterozygotes. Br. J. Cancer, 82,
1938–1945.
29. Fernet,M. et al. (2004) Cellular responses to ionising radiation of AT het-
erozygotes: differences between missense and truncating mutation carriers.
Br. J. Cancer, 90, 866–873.
30. Gatei,M. et al. (2003) Ataxia-telangiectasia-mutated (ATM) and NBS1-
dependent phosphorylation of Chk1 on Ser-317 in response to ionizing
radiation. J. Biol. Chem., 278, 14806–14811.
31. Lee,J.H. et al. (2004) Direct activation of the ATM protein kinase by the
Mre11/Rad50/Nbs1 complex. Science, 304, 93–96.
Received October 11, 2006; revised November 23, 2006;
accepted November 24, 2006
Finnish ataxia-telangiectasia mutations and breast cancer
1045
at Pomorska Akademia Medyczna on October 17, 2011
carcin.oxfordjournals.org
Downloaded from
Wyszukiwarka
Podobne podstrony:
Functional and Computational Assessment of Missense Variants in the Ataxia Telangiectasia Mutated (A
Newell, Shanks On the Role of Recognition in Decision Making
Morimoto, Iida, Sakagami The role of refections from behind the listener in spatial reflection
51 721 736 Evaluation of the Cyclic Behaviour During High Temperature Fatique of Hot Works
Explaining welfare state survival the role of economic freedom and globalization
86 1225 1236 Machinability of Martensitic Steels in Milling and the Role of Hardness
the role of women XTRFO2QO36SL46EPVBQIL4VWAM2XRN2VFIDSWYY
Illiad, The Role of Greek Gods in the Novel
The Role of the Teacher in Methods (1)
THE ROLE OF CATHARSISI IN RENAISSANCE PLAYS - Wstęp do literaturoznastwa, FILOLOGIA ANGIELSKA
The Role of Women in the Church
The Role of the Teacher in Teaching Methods
The role of the Victorian woman
the role of the victorian woman 2YEN3FEPRXWLO7M54JRW7LEE3Z4EI2JP533IAAA
the role of women
The Role of The Japanese Emperor in the Meiji Restoration
00 The role of E and P selecti Nieznany (2)
więcej podobnych podstron