
Combined osmotic and microwave-vacuum dehydration of apples
and strawberries
q
Ulrich Erle
*
, Helmar Schubert
Institute of Food Process Engineering, Karlsruhe Technical University, 76128 Karlsruhe, Germany
Received 1 June 2000; accepted 1 December 2000
Abstract
The combination of osmotic pre-treatment ± mainly in sucrose solutions ± and microwave-vacuum dehydration of strawberries
and apples has been studied. Water removal during osmotic treatment is accompanied by solute uptake from the osmotic solution.
The resulting changes in composition bring about better properties of the ®nal product in terms of structure and volume. Compared
to the solely microwave-vacuum dried samples, osmotic pre-treatment improved volume retention from 20% to 50% (strawberries)
and from approximately 20% to 60% (apples) based on the fresh volume. SEM pictures revealed that the cellular structure is also
preserved better, when osmotic pre-treatment is used. Gel formation between pectins, sucrose and in some cases, calcium ions is
believed to be the main cause of structure build-up. Vitamin C retention was around 60% with the microwave procedure applied
here. Ó 2001 Elsevier Science Ltd. All rights reserved.
Keywords: Volume retention; Vitamin retention; Structure; Gel formation; Pectin
1. Introduction
Osmotic dehydration is a very gentle method to re-
move water from plant tissues such as fruits or vegeta-
bles. While more than 50% of the water leaves the tissue
due to lower water activity in the surroundings, some
solutes from the osmotic solution are usually able to
penetrate the plant material despite the obstacles they
encounter in the form of cell walls and especially cell
membranes. These changes in composition can be ex-
ploited in order to create modi®ed properties of the ®nal
product. Unfortunately, the water activity attainable by
osmotic dehydration alone is still rather high. Further
means of water removal are therefore needed to ac-
complish the goal of shelf stability at room temperature.
In this study, microwave-vacuum dehydration has
been used to achieve water activity lower than 0.5. The
products obtained were of high quality concerning taste
and colour. Retention of vitamin C was measured, be-
cause the substance has a high nutritional value and its
absence is an indicator of thermal damage. Volume after
osmotic treatment alone and after combined treatment
was determined in order to demonstrate how the con-
ditions of osmotic treatment in¯uence both the osmotic
process itself and the properties of the ®nal product.
Measurements of the changes in sugar composition
during the osmotic process were taken to see, whether
the system can be treated like one of simple diusion.
2. Theory
The term `osmosis' refers to system of at least two
liquids with dierent solvent activities. These are sepa-
rated by a semipermeable membrane, i.e., a barrier
which lets the solvent pass but not the solutes. The result
is a ¯ow of solvent from the region of high to the region
of low activity.
In the case of fruits and vegetables the solvent is, of
course, water. Typical osmotically active substances in-
clude sugars, alcohols and salt (Barbosa-Canovas &
Vega-Mercado, 1996). Although the achievable molar
fractions are quite low, soluble starch is also an eective
osmotic agent (Isse & Schubert, 1995). In plant materi-
als, the cell membranes provide a ± more or less ±
Journal of Food Engineering 49 (2001) 193±199
www.elsevier.com/locate/jfoodeng
q
The ®rst part of this manuscript including Figs. 1±9 was
originally published in: Fito, P.; Chiralt, A.; Barat, J.M.; Spieû,
W.E.L. (2000). Osmotic Dehydration and Vacuum Impregnation:
Applications of New Technologies to Traditional Food Industries.
Technomic Publishing, Lancaster, USA.
*
Corresponding author. Tel.: +49-721-608-4366; fax: +49-721-
694320.
E-mail addresses: ulrich.erle@lvt.uni-karlsruhe.de, ulrich.erle@
merck. de (U. Erle).
0260-8774/01/$ - see front matter Ó 2001 Elsevier Science Ltd. All rights reserved.
PII: S 0 26 0 - 8 7 7 4 ( 0 0 ) 0 0 20 7 - 7
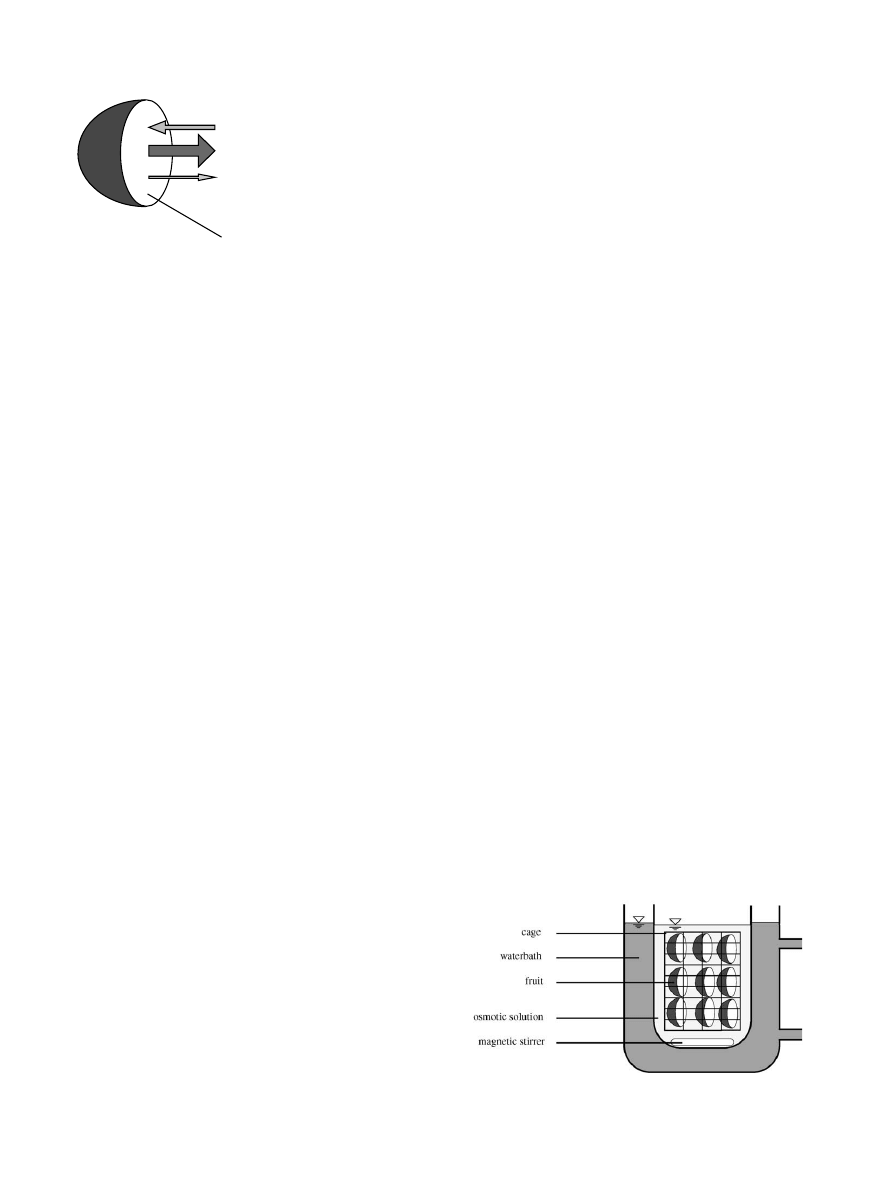
semipermeable barrier. In fact, some part of the solutes
is usually able to penetrate the tissue. The material may
also lose a portion of its own solutes (vitamins, volatiles,
minerals. . .). Fig. 1 gives an overview of these transport
phenomena.
Owing to the low boiling point at low pressures,
vacuum application oers the bene®ts of dehydration at
temperatures, where the thermal damage is practically
non-existent. At 5 kPa, the pressure applied in this
study, the boiling point of pure water is 32.9°C. As long
as there is enough water in the tissue, this boiling point
can only be exceeded minimally due to dissolved sub-
stances. In the ®nal stages of microwave drying, tem-
perature may reach 80°C, but thermal damage in this
period is still relatively low, because the heat sensitivity
decreases with decreasing water content. The use of
microwaves overcomes the usual problem of poor heat
transfer in vacuum drying. In microwave-vacuum dry-
ing, heat is not transferred to but generated in the tissue.
This allows for energy transfer rates much higher than in
conventional drying operations, especially in the falling
rate period (Roussy & Pearce, 1995). The absorption of
microwaves by a sample depends on its dielectric
properties, which are positively correlated with the wa-
ter content. That is why, to a certain extent, the distri-
bution of power in microwave drying is self controlled:
those areas which received more power than others dry
quicker and will therefore absorb less power from then
on. However, when the power level is set too high,
overheating and burning of dry areas, a so-called
`thermal runaway', may occur (Buer, 1993).
The application of an osmotic treatment prior to
microwave-vacuum drying combines the advantages of
both unit operations in a unique way: since no phase
transition takes place in osmotic dehydration, energy
consumption is especially low, even if the diluted solu-
tion needs to be reconcentrated by evaporation. Mi-
crowaves require electricity, a relatively expensive form
of energy, but they are only employed in the ®nal stages
of drying, where they can be used more eciently than
hot air (Gunasekaran, 1999).
Structure and volume of the product may suer
during microwave-vacuum dehydration. Osmotic pretr-
eaments provide a tool for incorporating sucrose or
other solutes in the food, which may already contain
pectins. These are known to form gels with sucrose and
calcium ions (Pilnik, 1980; Glenn & Poovaiah, 1990).
The resulting improvements of structure and volume are
the main subject of this study. Another bene®cial eect
is that the addition of sugars in general means that less
water needs to be removed for shelf stability. When ions
are used in the osmotic solution, they will normally enter
the tissue, thereby increasing its dielectric loss factor,
which makes microwave drying more ecient.
3. Material and methods
3.1. Osmotic treatment of apples
Apples (`Golden Delicious') were cored, peeled and
cut in 12slices, which were treated osmotically. Ap-
proximately 400 g of apple slices (12±18 cm
3
) were put
into a stirred 60% (w/w) sucrose solution (see Fig. 2).
When calcium chloride was added to the solution, su-
crose concentration was decreased accordingly, so as to
keep the water concentration constant at 40% (w/w).
The ratio of apples to solution was 1:9. Osmotic treat-
ment was carried out for durations ranging from 2±25 h.
Temperatures were between 20°C and 70°C. Concen-
tration of calcium chloride was varied between 0% and
6%. After treatment, samples were dipped into water ®ve
times, blotted in kitchen paper, weighed and used for
measurements or microwave-vacuum drying.
3.2. Osmotic treatment of strawberries (for sugar and
vitamin C measurements)
Measurements of sugar concentration and vitamin C
tend to be quite scattered when they are conducted with
dierent batches or with the same batch, but at dierent
ripeness of the fruits. Hence, a bigger vessel for osmotic
dehydration has been used in these experiments. It is
capable of holding more than 20 samples of 100 g from
one batch resulting in much reduced scattering of the
data compared to earlier measurements, in which a new
water
dissolved substances:
sucrose
calcium
dissolved substances:
sugars
minerals
colour
volatiles
piece of fruit
semipermeable membrane(s)
Fig. 1. Mass transfer during osmotic treatment.
Fig. 2. Set-up for osmotic treatment of 400 g of fruit.
194
U. Erle, H. Schubert / Journal of Food Engineering 49 (2001) 193±199
batch of fruit had to be used for each duration of os-
motic treatment. One objective here was to detect pos-
sible enzymic activity due to osmotic treatment in
sucrose solution, which would cause changes in the
sugar composition of the strawberries. In addition, the
sucrose uptake in time had to be documented.
Twenty-two samples of 100 1 g of medium sized
strawberry halves (4±8 cm
3
) were put in nets and dried in
a stirred 60% sucrose solution (20 kg) at 20°C. After
treatment, samples were dipped into water three times,
blotted in kitchen paper, weighed and frozen for later
analysis or microwave-vacuum dried for vitamin C
measurements of the ®nal product. Three more samples
were just dipped in a 60% sucrose solution and then
treated like the other samples to serve as reference.
3.3. Osmotic treatment of strawberries (for volume
measurements)
The small vessel (see Fig. 2) was used for the osmotic
treatment of strawberries in those experiments con-
cerning the volume retention. Four hundred grams of
medium-sized strawberry halves were treated in 60%
sucrose solution for 3±22 h at 20°C.
3.4. Microwave-vacuumdrying
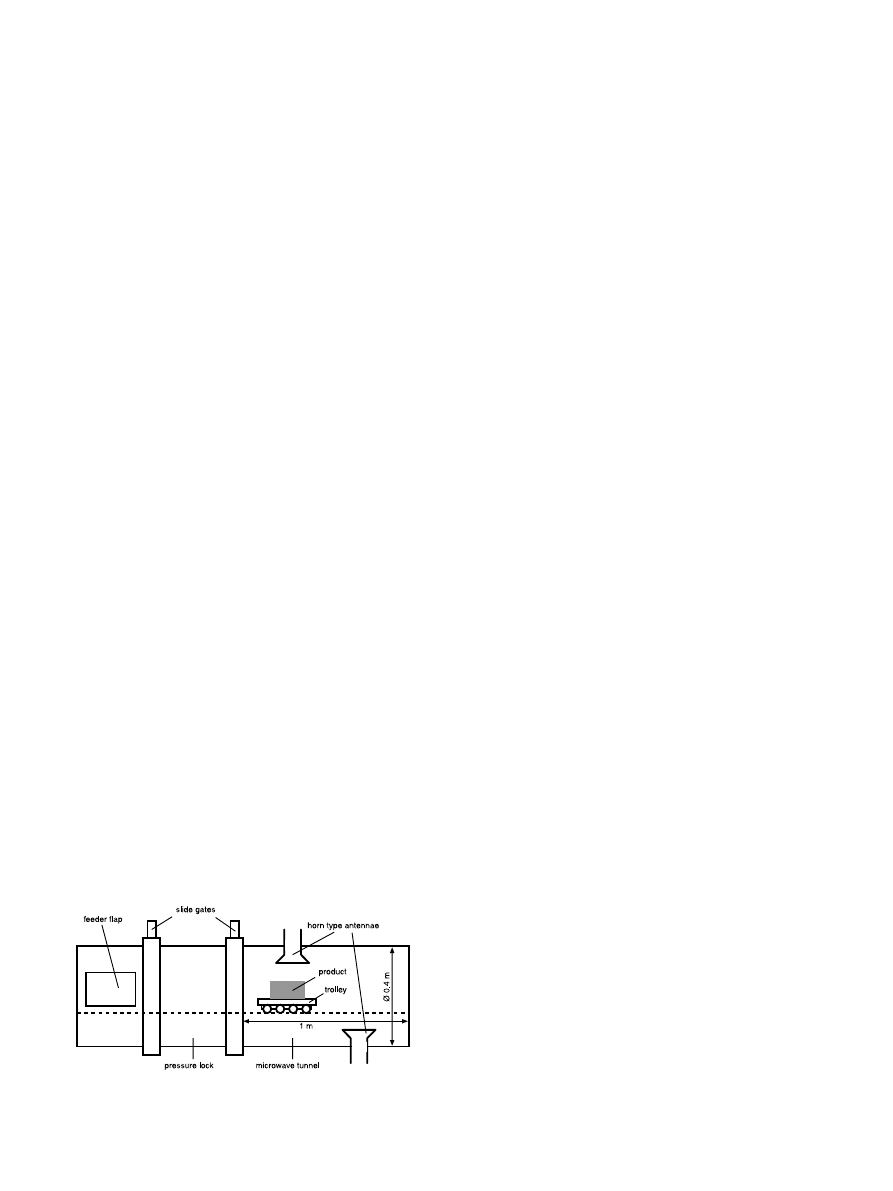
Microwave drying of the fresh and osmotically
treated fruits took place in a pilot-scale microwave
plant, designed to simulate an industrial microwave
tunnel (see Fig. 3). The pieces were put on a tray which
allows the steam to exit in all directions. The trolley goes
back and forth, and the magnetrons are only switched
on when it comes near the corresponding horn antenna,
so the microwaves are used in a pulsed way. Since mi-
crowave power absorption depends mainly on the
amount of water in the samples, the microwave dryer
was always fed with samples containing 140 5 g of
water, regardless of the percentage of water removed in
the osmotic process. This makes the experiments com-
parable. However, one of the results in earlier experi-
ments was that the osmotic pre-treatment made
microwave drying more ecient both in terms of re-
moved water and achieved water activity. The main
reasons for this are higher dielectric losses caused by the
ions and sucrose (Padua, 1993) as well as the better
microwave absorption of less shrunk objects. As a re-
sult, the following microwave programs were applied:
· Strawberries: 390 W for 37 min plus 195 W for 15
min;
· Apples (osmotically treated at 20°C without calcium):
390 W for 30 min plus 195 W for 39 min;
· Apples (osmotically treated at temperatures higher
than 20°C or with calcium): 390 W for 21 min plus
195 W for 13 min;
· Untreated, fresh apple and strawberry pieces needed
longer microwave drying. Water activities lower than
0.5 were achieved in all cases. Pressure was always
5 kPa.
3.5. Measurements of sugar and vitamin C
Frozen samples were homogenized in a laboratory
mixer together with 100 g of ice and 50 ml of 15% meta-
phosphoric acid. The slurry was poured into a ¯ask and
its pH was altered to 3.5±4 by potassium hydroxide. The
¯ask was then ®lled with water up to 500 ml. The con-
tents of the ¯ask were ®ltered and used for the following
measurements.
Fructose, glucose and sucrose were determined by
HPLC. Vitamin C in strawberries was measured by an
enzymic test (Boehringer no. 409 677). As the vitamin
content in the apples used here proved to be rather low,
a dierent method had to be chosen: Vitamin C
(ascorbic acid + dehydroascorbic acid) in fresh, osmoti-
cally treated and microwaved apples was measured by a
chemical method involving titration with dichlorphe-
nolindophenol-sodium. A 1% solution of oxalic acid was
used in the homogenizer to inhibit further degradation
of the vitamin C. After homogenization, the slurry was
®ltered, and the chemical procedure was applied to the
®ltrate.
3.6. Measurements of calcium in apple tissue
Six grams of fresh apple tissue or a smaller amount of
osmotically treated apple tissue (equalling 6 g before
osmotic treatment) from ®ve dierent slices were meshed
and boiled for 2h in a mixture of 21 ml concentrated
HCl and 7 ml concentrated HNO
3
. This mixture was
®ltered, and a 3.5% HNO
3
solution was added up to 100
ml. Calcium content in apple tissue was determined by
Atomic Absorption Spectroscopy (Perkin Elmer 1100 B)
at 422.7 nm.
3.7. Volume measurements
Volume of the fresh and osmotically treated straw-
berries and apples was determined by measuring their
Fig. 3. Microwave pilot plant.
U. Erle, H. Schubert / Journal of Food Engineering 49 (2001) 193±199
195
buoyancy in water, while the volume of dry samples was
found through measuring the displacement of glass
spheres of known bulk density.
4. Results and discussion
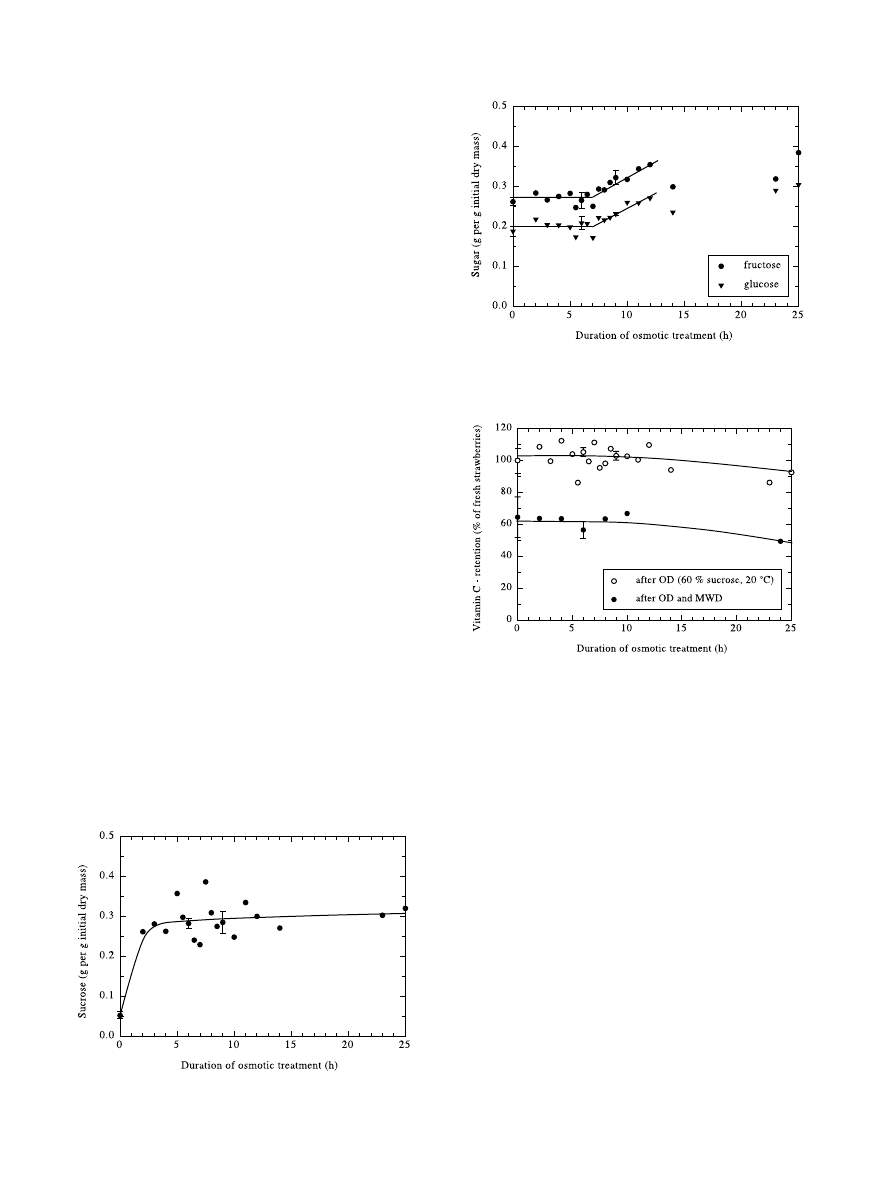
The gain of sucrose during osmotic dehydration of
strawberries is depicted in Fig. 4. The ®rst triple point at
zero hours osmotic treatment ± the samples were just
dipped in the sucrose solution and then washed imme-
diately ± gave a starting point of 5% sucrose based on
the initial dry mass. Most of the following sucrose gain
of approximately 25±30% (initial dry basis) happened
within the ®rst two hours.
The evolution of the fructose and glucose content is
presented in Fig. 5. There was no signi®cant increase of
the two sugars in the ®rst few hours. After approxi-
mately 7 h, an upward trend for both glucose and
fructose started. This was most likely caused by hydro-
lysis of sucrose. Since the sucrose content remained more
or less constant in that period of time, the conclusion is
that the rate of sucrose conversion into glucose and
fructose was the same as the rate of sucrose entering the
tissue. The ¯ow of sucrose seemed to have stopped (see
Fig. 4), but in reality it had not. Models describing os-
motic dehydration should take into account that enzy-
mic reactions may ± after a few hours ± in¯uence the
sugar composition and therefore the whole process.
Preservation of vitamin C is the subject of Fig. 6.
During the osmotic treatment (upper curve) a loss of a
few percent ± comparable to typical losses during storage
at 20°C ± occurred for long durations. After microwave-
vacuum drying with the procedure described earlier,
approximately 60% of the vitamin was still detected, re-
gardless of the duration of osmotic treatment. Single
experiments with more gentle procedures (not reported
here) indicate that preservation of the vitamin can still be
improved, but only at the price of longer drying times.
Fig. 7 shows that the apples exhibited virtually no
loss of vitamin C during osmotic treatment. The ®nal
vitamin C content was around 60% with no obvious
correlation to the duration of the osmotic treatment.
Like for the strawberries, this value is only valid for the
microwave procedure applied here.
The concentration of vitamin C in these apples was
quite low even in the fresh material (25 mg/100 g of
initial dry matter), which is why the enzymic test had to
be replaced by a chemical method. In this particular
case, the retention of the vitamin is not very important
for nutritional reasons, but still serves as an indicator of
thermal damage.
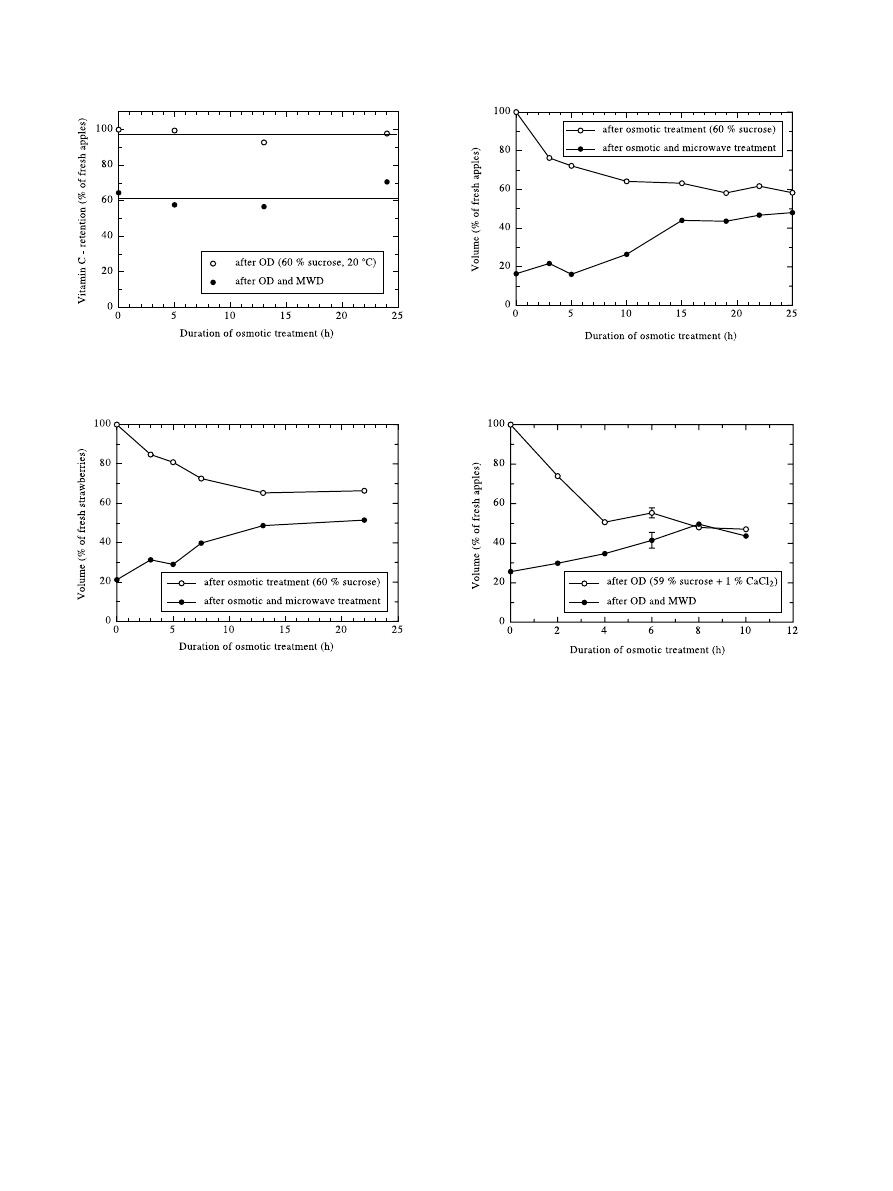
In Fig. 8, the upper curve mainly represents shrinkage
caused by water loss during osmotic dehydration of
strawberries. The lower curve shows how much an os-
motic pre-treatment can in¯uence the volume of the ®nal
product. Strawberries which stayed in the sucrose solu-
tion for 22 h took up a volume higher by a factor of 2.5
compared to those solely microwave-dried. It is appar-
ent that this increase cannot be explained by the volume
Fig. 4. Sucrose uptake of strawberries in 60% sucrose solution at 20°C.
Fig. 5. Evolution of fructose and glucose during osmotic dehydration
of strawberries in 60% sucrose solution at 20°C.
Fig. 6. Vitamin C retention during osmotic and combined drying of
strawberries.
196
U. Erle, H. Schubert / Journal of Food Engineering 49 (2001) 193±199
of the extra sucrose itself. Samples without pre-treat-
ment were also notably softer than those with a long
osmotic step. The latter appeared more crispy and
brittle. It is very likely to be the interaction of the su-
crose with the pectins in the strawberries that has caused
this change in structure and volume.
Apple slices were also treated osmotically under the
same conditions as strawberries. The result ± given in
Fig. 9 ± was very similar to that of the strawberries.
Again, the volume of the pieces after microwave drying
could be increased by a factor of up to 2.5 (lower curve).
Fig. 10 displays how the samples behave at various
durations of osmotic treatment, when temperature is
50°C, and the concentration of calcium chloride is 1%.
The main conclusion from this curve is especially of
practical relevance as it aects the design of industrial
equipment. Under these conditions, it takes approxi-
mately 8 h to reach the maximum eect concerning the
®nal volume. This is much quicker than at 20°C without
calcium (Fig. 9). Then, 15 h were needed for maximum
volume. Another interesting aspect here is that the re-
moval of a great portion of the water takes place even in
the ®rst four hours, but the mechanism responsible for
structure build-up takes twice as long.
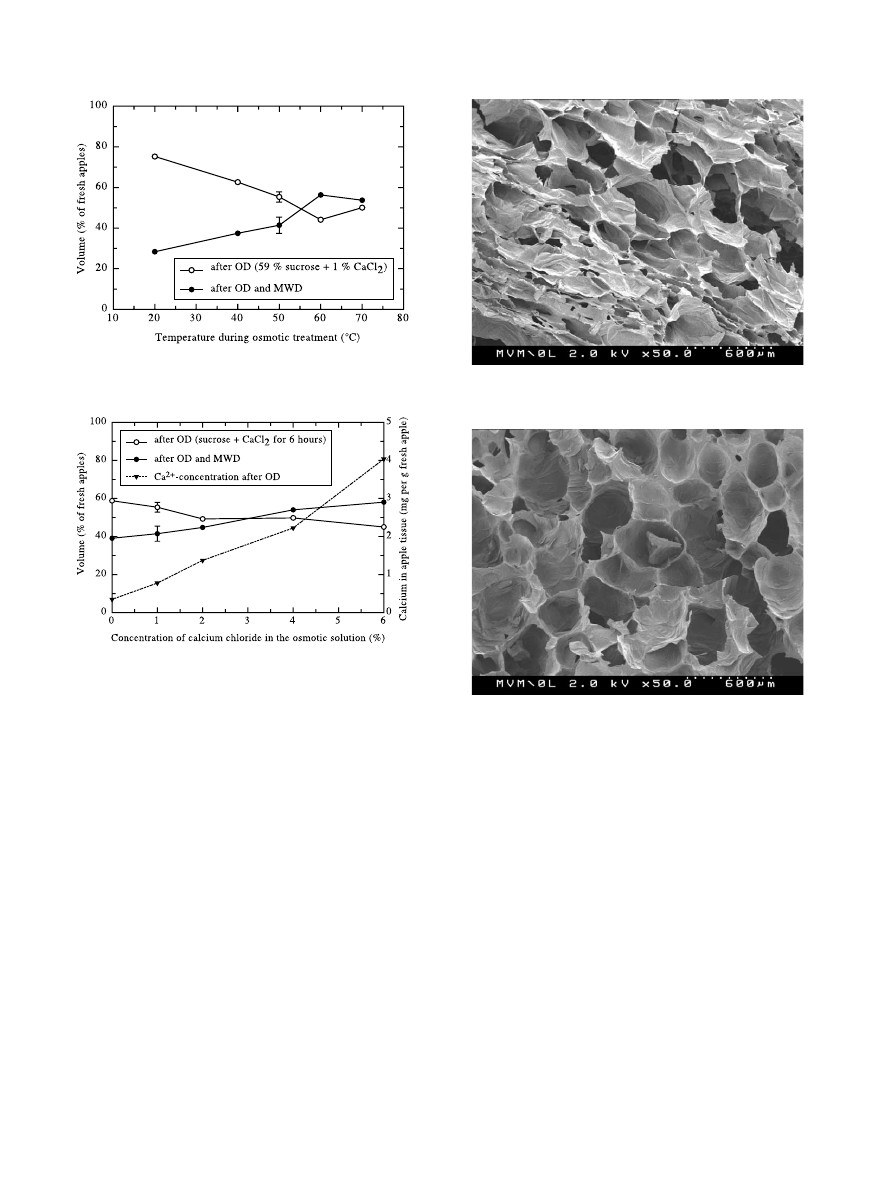
Fig. 11 shows the eect of various temperatures on
the volume of the apples at constant calcium chloride
concentration of 1% and constant duration of 6 h. Like
before, the upper curve gives the volume after osmotic
treatment alone, and the lower curve is for the ®nal
volume. The error bars represent the standard deviation
of three measurements. There is a clear trend concerning
the speed of water removal: going to higher tempera-
tures, the upper curve drops, indicating accelerated
water transport. Since enhanced uptake of sucrose and
calcium are also linked to higher temperatures, the
volume of the ®nal product, in which pectins, sucrose
and calcium are assumed to take part in gel formation,
can be increased considerably.
In Fig. 12the concentration of calcium chloride in the
osmotic solution was varied at constant temperature
(50°C) and duration (6 h). As mentioned before, sucrose
concentration was 60% minus calcium chloride con-
Fig. 9. Shrinkage during osmotic and combined drying of apples at
20°C.
Fig. 10. Shrinkage during osmotic and combined drying of apples at
50°C.
Fig. 8. Shrinkage during osmotic and combined drying of strawberries
at 20°C.
Fig. 7. Vitamin C retention during osmotic and combined drying of
apples.
U. Erle, H. Schubert / Journal of Food Engineering 49 (2001) 193±199
197
centration. Since calcium chloride, which replaced the
same amount of sucrose, has a higher osmotic activity
per gram than sucrose, the volume of the samples after
osmosis has a slight downward trend. However, the
volume of the ®nal product could clearly be enhanced by
the calcium. For high concentrations of calcium the
samples even expanded in the microwave drier. This can
be explained by a certain pressure build-up in the tissue
as steam is created rapidly (mainly in the ®rst half of the
drying time). Of course, pressure is also generated in
those samples, which are not pre-treated. The key
question is whether the material is stable enough to
withstand the contracting force that occurs in the ®nal
stages of drying, when the expanding force of the steam
ceases. Obviously, the added calcium renders this extra
stability. The texture of those samples treated with high
concentrations of calcium was especially rigid and brit-
tle; it resembled a freeze dried product.
In an attempt to increase the volume of dried apple
further, the following two osmotic treatments were also
tested: 8 h at 60°C in 60% sucrose and in 54% sucrose
plus 6% calcium chloride, respectively. There was no
additional improvement. The ®nal volume was 59% of
the fresh volume in both cases.
Fig. 13 is an SEM picture of an untreated apple slice
after microwave-vacuum drying. The surface shown was
created by breaking the sample in liquid nitrogen after
drying. For comparison, Fig. 14 demonstrates the better
retention of volume and shape at the cellular level in the
osmotically pre-treated sample (60°C for 6 h with 1%
calcium chloride).
5. Conclusions
Microwave-vacuum drying of osmotically pre-treated
fruits combines the bene®ts of both unit operations.
Selecting the conditions during osmotic treatment oers
the possibility of in¯uencing both the eciency of mi-
crowave dehydration and the properties of the ®nal
product. High-quality products in terms of colour, taste,
Fig. 13. Cell structure of microwave-vacuum dried apple without pre-
treatment.
Fig. 14. Cell structure of microwave-vacuum dried apple with pre-
treatment.
Fig. 11. Shrinkage during osmotic and combined drying of apples for
6 h.
Fig. 12. Shrinkage during osmotic and combined drying of apples at
50°C.
198
U. Erle, H. Schubert / Journal of Food Engineering 49 (2001) 193±199

vitamin C content, structure and volume can be ob-
tained. Vitamin C retention was around 60% for apples
and strawberries with the microwave program used in
this study. In the combined process, volume was pre-
served by up to 60% of the fresh apples and up to 50% of
the fresh strawberries, respectively.
Acknowledgements
The authors wish to thank the Max-Buchner-For-
schungsstiftung for the support of this research.
References
Barbosa-Canovas, G. V., & Vega-Mercado, H. (1996). Dehydration of
foods. New York: Chapman and Hall.
Buer, C. R. (1993). Microwave cooking and processing: engineering
fundamentals for the food scientist. New York: Van Nostrand
Reinhold.
Glenn, G. M., & Poovaiah, B. W. (1990). Calcium mediated
postharvest changes in texture and cell wall structure and compo-
sition in `Golden Delicious' apples. Journal American Society of
Horticulture Science, 115(6), 962±968.
Gunasekaran, S. (1999). Pulsed microwave-vacuum drying of food
materials. Drying Technology, 17(3), 395±412.
Isse, M.G., Schubert, H. (1995). Dissolved starch as an osmotic
dehydrating agent of fruit and vegetables. In F. Meuser, D. J.
Manners, & W. Seibel, (Eds.), Progress in plant polymeric carbo-
hydrate research. Hamburg: Behr`s Verlag.
Padua, G. W. (1993). Microwave-heating of agar gels containing
sucrose. Journal of Food Science, 58(6), 1426±1428.
Pilnik, W. (1980). Pektine und Alginate. In Gelier- und Verdickung-
smittel in Lebensmitteln (Gelling and thickening agents in foods).
Zurich, Switzerland: Forster Verlag AG.
Roussy, G., & Pearce, J. A. (1995). Foundations and industrial
applications of microwaves and radio frequency ®elds. Great Britain:
Wiley.
U. Erle, H. Schubert / Journal of Food Engineering 49 (2001) 193±199
199
Wyszukiwarka
Podobne podstrony:
więcej podobnych podstron