
Yeast 14, 1267–1283 (1998)
Comparison of Expression Systems in the Yeasts
Saccharomyces cerevisiae, Hansenula polymorpha,
Klyveromyces lactis, Schizosaccharomyces pombe and
Yarrowia lipolytica. Cloning of Two Novel Promoters
from Yarrowia lipolytica
SVEN MU
} LLER
2
, THOMAS SANDAL
1
*, PETER KAMP-HANSEN
1
AND HENRIK DALBØGE
1
1
Microbial Discovery I, Novo Nordisk A/S, Novo Alle´, Building 1.B1.20, DK-2880, Bagsværd, Denmark
2
Department of Life Science and Chemistry, Roskilde University, Building 17.2, PO Box 260, DK-4000 Roskilde,
Denmark
We have compared expression systems based on autonomously replicating vectors in the yeasts Saccharomyces
cerevisiae, Schizosaccharomyces pombe, Kluyveromyces lactis, Hansenula polymorpha and Yarrowia lipolytica in order
to identify a more suitable host organism for use in the expression cloning method (Dalbøge and Heldt-Hansen,
1994) in which S. cerevisiae has traditionally been used. The capacity of the expression systems to secrete active
forms of six fungal genes encoding the enzymes galactanase, lipase, polygalacturonase, xylanase and two cellulases
was examined, as well as glycosylation pattern, plasmid stability and transformation frequency. All of the examined
alternative hosts were able to secrete more active enzyme than S. cerevisiae but the relative expression capacity of the
individual hosts varied significantly in a gene-dependent manner. One of the most attractive of the alternative host
organisms, Y. lipolytica, yielded an increase which ranged from 4·5 times to more than two orders of magnitude. As
the initially employed Y. lipolytica XPR2 promoter is unfit in the context of expression cloning, two novel promoter
sequences for highly expressed genes present in only one copy on the genome were isolated. Based on sequence
homology, the genes were identified as TEF, encoding translation elongation factor-1á and RPS7, encoding
ribosomal protein S7. Using the heterologous cellulase II (celII) and xylanase I (xylI) as reporter genes, the e
ffect of
the new promoters was measured in qualitative and quantitative assays. Based on the present tests of the new
promoters, Y. lipolytica appears as a highly attractive alternative to S. cerevisiae as a host organism for expression
cloning. GenBank Accession Numbers: TEF gene promoter sequence: AF054508; RPS7 gene promoter sequence:
AF054509.
1998 John Wiley & Sons, Ltd.
— non-Saccharomyces yeasts; heterologous gene expression; autonomously replicating expression
vectors; selective promoter identification
INTRODUCTION
Isolation of enzyme genes from filamentous fungi
by use of the expression cloning method (Dalbøge
and Heldt-Hansen, 1994) has proved to be a very
strong alternative to traditional cloning methods
based on amino acid sequence information. So far,
approximately 200 genes have been cloned in our
laboratory, of which several have been character-
ized (Christgau et al., 1994, 1995, 1996; Kofod
et al., 1994; Draborg et al., 1995, 1996; Kauppinen
et al., 1995). Successful use of the method
demands: (i) e
ffective methods for generation of
full-length cDNAs; (ii) sensitive and reliable
enzyme assays; and (iii) a host organism which
e
fficiently secretes active forms of the cloned gene
products. The use of yeast as a host organism is
convenient because of its close evolutionary rela-
tionship with fungi. In addition, as a unicellular
microorganism, yeast o
ffers the advantages of
bacterial systems with regard to ease of manipula-
tion and growth conditions. So far, Saccharomyces
cerevisiae has been used as the host organism for
isolation of enzyme genes by expression cloning
but S. cerevisiae has been found to have certain
*Correspondence to: T. Sandal.
CCC 0749–503X/98/141267–17 $17.50
1998 John Wiley & Sons, Ltd.
Received 24 March 1998
Accepted 28 June 1998

limitations as a host for heterologous gene expres-
sion. In general, product yields are very low, with a
maximum of 1–5% of total protein, and plasmid
instability, hyperglycosylation and retention of
the proteins within the periplasmic space have
been observed (for references, see Buckholz
and Gleeson, 1991). Even though the use of S.
cerevisiae as a host organism has resulted in the
cloning of a large number of genes, the outlined
limitations of this host organism indicate that the
detection of some of the cloned heterologous
genes fails during the plate screening procedures.
Furthermore, the low expression level constitutes a
bottleneck for the expression cloning method, as it
necessitates reintroduction of the isolated genes
into a more e
ffective expression system prior to the
initial characterization of the products. Clearly,
it would be an improvement of the expression
cloning method if the host organism more e
ffi-
ciently secreted an active form of the heterologous
gene product. S. cerevisiae has been developed as a
production system for many di
fferent proteins (for
references, see Gellissen and Hollenberg, 1997) but
the increased product yield and improved quality
of the recombinant products have been achieved
by the introduction of tailor-made mutant strains
in which relevant steps of protein synthesis or
secretion have been altered. This product-specific
boosting of gene expression is not an option for
improvement of the expression cloning method in
which cDNAs from various donors encoding a
broad range of enzymes are introduced into the
host organism. Rather, it would be an improve-
ment of the expression cloning method to intro-
duce a more tolerant host organism which secretes
active forms of a broad range of heterologous gene
products more e
fficiently.
A growing number of non-Saccharomyces yeasts
have become accessible as expression systems
for heterologous gene products. The performance
of these alternative host organisms, including
Hansenula polymorpha, Klyveromyces lactis, Pichia
pastoris, Schizosaccharomyces pombe, Schwannio-
myces occidentalis and Yarrowia lipolytica, has
been reviewed in relation to S. cerevisiae (see
e.g. Buckholz and Gleeson, 1991; Gellissen and
Hollenberg, 1997) but the relative tolerance of
these organisms as hosts for expression of a broad
range of identical heterologous gene products has
not been tested and compared. In order to improve
the expression cloning method, we examined the
potential of the yeasts H. polymorpha, K. lactis, S.
pombe and Y. lipolytica, in which expression of
heterologous gene products is possible by use
of autonomously replicating expression vectors
(Gleeson et al., 1986; Bro¨ker and Ba¨uml, 1989;
Fleer et al., 1991; Fournier et al., 1991), with focus
on their ability to secrete active forms of six fungal
test gene products.
In this study we report that Yarrowia lipolytica
emerged as one of the most attractive alternative
host organisms. However, the homologue XPR2
promoter employed in the initial Y. lipolytica ex-
pression system is unsuitable in the context of
expression cloning, mainly because full induction
requires high levels of peptone in the culture
medium (Ogrydziak and Scharf, 1982). A search
for more attractive Y. lipolytica promoters was
therefore initiated. In the context of the expression
cloning method, an ideal promoter is characterized
by strength, activity in a medium suitable for
product recovery and inducibility. A strong pro-
moter is a necessary prerequisite for a high expres-
sion level and, with a very low copy number of the
centromere-linked ars18-based expression vectors
(Fournier et al., 1991; Vernis et al., 1997), this
character is even more important when Y. lipo-
lytica is used as the host organism. A tightly
regulated promoter makes it possible to separate
the growth stage from the expression stage,
thereby enabling expression of products which are
known to inhibit cell growth.
We report the cloning and the sequences of the
translation elongation factor-1á (TEF) and the
ribosomal protein S7 (RPS7) gene promoters from
Y. lipolytica. Based on tests of the novel promoters
with two heterologous reporter genes, Y. lipolytica
appears as a highly attractive alternative host
organism for use in expression cloning.
MATERIALS AND METHODS
Strains, media and growth conditions
Bacterial and yeast strains are described in
. The various yeast transformants were
precultured in selective synthetic complete (SC)
media (Sherman, 1991) containing 2% glucose and
lacking either uracil or leucine. Of the non-
selective inducing media, 100 ml were inoculated
with precultures to an optical density at 600 nm
(OD
600
) of 0·1. Inducing media for the various
transformants were as follows: S. cerevisiae was
grown in 2
YP with 4% galactose; S. pombe and
K. lactis were grown in 2
YP with 4% glucose; H.
polymorpha was grown in a YP-derived medium
1268
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

Table 1. Bacterial strains, yeast strains and expression vectors used in the study.
Bacterial strains
Source
MC1061
Meissner et al., 1987
DH10B
Gibco BRL
SJ2
A C600 derivate (Raleigh et al., 1988) modified at Novo Nordisk A/S
Yeast strains
Features
Source
Saccharomyces cerevisiae W 3124
Mat a ura 3-52 leu 2–3,112 his 3-Ä200
pep4-1137 Äprc1::HIS3 prb1::LEU 2 cir
+
J. Winther, Carlsberg Research Laboratory,
Department of Yeast Genetics, Denmark
Hansemula polymorpha A16
A derivate of the wild-type strain CBS4732
P. E. Sudbury, University of She
ffield, UK
Kluyveromyces lactis MW98-8C
Mat á uraA arg lys K
+
pKD1
0
M. Wesolowski-Louvel, Institut Curie,
Centre Universitaire, Orsay, France
Schizosaccharomyces pombe 972
h ura4-294
R. Egel, Department of Genetics,
University of Copenhagen, Denmark
Yarrowia lipolytica P
0
1d
A derivative of W29 Mat A: ura 3-302
leu 2-270 xpr 2-322
C. Gaillardin, Centre de Biotechnologies
Agro-Industrielles, Thiverval-Grignon, France
Expression
vectors
Host
Size
(kb)
Homologue
promoter
Replicative
element
Selection
marker
in yeast
Original source/
modifications
pYES 2·0
Saccharomyces cerevisiae
5·9 GAL1-inducible
2 ì
URA3 Invitrogen U.S.A.
pC4
Saccharomyces cerevisiae
5·9 GAL1-inducible
2 ì
URA3 Invitrogen U.S.A.
Introduction of an SfiI restriction site
pK2
Kluyveromyces lactis
7·0 LAC4-constitutive Structurally related to 2 ì URA3 pKD1 (Fleer et al., 1991)
pY1
Yarrowia lipolytica
7·8 XPR2-inducible
ars18
LEU2
pINA532 (C. Gaillardin)
pH2
Hansenula polymorpha
14·0 MOX-inducible
2 ì
LEU2
YEp13 (Gleeson et al., 1986)
pP3
Schizosaccharomyces pombe 7·1 ADH-constitutive
ars and stb
URA3 pMB332 (Bro¨ker and Ba¨uml, 1989)
pY3
Yarrowia lipolytica
7·9 XPR2-inducible
ars18
LEU2
As pY1, except no SfiI site is
present in the polylinker
1269
1998
John
Wiley
&
Sons,
L
td.
Yeast
14,
1267–1283
(1998)

(‘BMMY’, Pichia Expression Kit, from Invitro-
gen) containing 2% methanol; Y. lipolytica was
grown in 0·2% yeast extract, 0·1% glucose and 10%
proteose peptone in 50 m
NaHPO
4
, pH 6·8 (a
modification of the YPDm medium; Nicaud et al.,
1989) when the test gene expression was based on
the XPR2 promoter. When Y. lipolytica test gene
expression was based on the novel promoters,
transformants were grown in SC-leu medium
containing 2% glucose. All transformants were
cultivated in 0·5 l shake flasks at 30
C. Solid media
(see
) were made with 1% agarose and
0·1% azurine dyed and cross-linked (AZCL) HE-
cellulose or Birch-xylan substrate (MEGAZYME).
Plasmids, test genes and transformation procedures
Expression vectors of the various yeasts are
described in
. Furthermore, plasmid pYES
2·0 (Invitrogen) was used for cloning of Y. lipo-
lytica cDNAs, pSJ1678 (Novo Nordisk A/S) for
cloning of Y. lipolytica genomic DNA and pUC19
(Yanish-Perron et al., 1985) was used to enable
sequence determination of positives from the Y.
lipolytica genomic library originally cloned in
pSJ1678.
Test genes and donor organisms are described
in
. The test genes were cloned in the
pC4, pK2, pY1, pH2 and pP3 expression vectors
) as 5
–SfiI 3–NotI fragments. The celII
and xylI reporter genes were cloned in pY3 (and
modified editions of this vector including the
new Y. lipolytica promoters) as 5
–BamHI 3–NotI
fragments.
Plasmids were transformed into electrocompe-
tent E. coli strains MC1061 and SJ2, as described
(Meissner et al., 1987). Electrocompetent DH10B
cells were transformed as recommended by the
manufacturer. Transformation of S. cerevisiae, K.
lactis and Y. lipolytica with self-replicative expres-
sion vectors was carried out by electroporation
(Becker and Guarente, 1991). H. polymorpha was
transformed by use of the protoplast method
(Gleeson et al., 1986). Intact S. pombe cells were
transformed as described (Bro¨ker, 1987).
Measurement of enzymatic activity
Activity determination of Cellulase I, Cellulase
II, Galactanase I and Xylanase I was performed in
liquid assays. The degradation of AZCL substrates
(HE-cellulose, Arabinogalactan and Birch-xylan
(MEGAZYME)) was measured spectrophoto-
metrically at OD
620
and related to the correspond-
ing activity of the native enzyme. Supernatants
were incubated, shaking, at 40
C in: 0·04 citrate/
phosphate, pH 5·5 (Cellulase I); 0·04
Tris,
pH 7·5 (Cellulase II); 0·04
citrate/phosphate,
pH 4·5 (Galactanase I); or 0·04
Tris, pH 7
(Xylanase I) in the presence of 0·16% substrate.
Polygalacturonase I and Lipase I activity was
measured on substrate-containing agarose plates
on which the area of the clearing zones was related
to a titration of the respective native enzyme.
Polygalacturonase I plates: 1% agarose, 0·1
citrate/phosphate, pH 4·5 and 1% Obipektin (DE
35%, NN Switzerland). Supernatants were in-
cubated for 24 h at 30
C prior to precipitation
with 1% mixed alkyltrimethylammoniumbromide
(MTAB) (Sigma
) solution at RT. Lipase I plates:
2% agarose, 0·1
Tris, pH 9·5, 0·1 CaCl
2
, 0·5%
olive oil and 1% Triton X-100. Supernatants were
incubated for 24 h at 30
C. For each construction,
supernatants from two or three independently
inoculated cultures were assayed in duplicate. Re-
producibility was generally better than 8% be-
tween duplicates. Data from repeated experiments
generally varied less than 15%.
Electrophoresis and Western blotting
SDS–PAGE was performed essentially as
described by Laemmli (1970). Molecular mass
markers ranging from 6 to 98 kDa were used
(Pharmacia). 25 ìl supernatant aliquots of the
samples used for measuring of enzymatic activity
were loaded.
SDS–PAGE gels were blotted to polyvinylidine
difluoride membranes (‘Immobilon’, Millipore
Corp.) by semi-dry electrobotting (Kyhse-
Andersen, 1984). Unreacted binding sites were
blocked by incubation for 30 min at RT in 0·05
Tris, pH 7·5, 0·15
NaCl, 0·1% Tween 20 contain-
ing 2% bovine serum albumin. For immunoblot-
ting, the membranes were incubated for 60 min at
Table 2. Test genes used.
Test genes
Origin
Cellulase I
Aspergillus aculeatus
Cellulase II
Humicola insolens
Galactanase I
Aspergillus aculeatus
Xylanase I
Humicola insolens
Polygalacturonase I
Aspergillus aculeatus
Lipase I
Thermomyces lanuginosus
1270
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

RT with a polyclonal rabbit antiserum raised
against the tested enzymes diluted 1:1000 in 0·05
Tris, pH 7·5, 0·15
NaCl, 0·1% Tween 20. Bound
rabbit antibodies were visualized by incubation
for 60 min at RT with alkaline phosphatase
conjugated goat anti-rabbit antibodies (DAKO
A/S, Denmark), diluted in 0·05
Tris, pH 7·5,
0·15
NaCl, 0·1% Tween 20 to 0·6 ìg/ml and
development in 10 ml ALP bu
ffer (150 m Tris–
Cl, pH 9·5, 100 m
NaCl, 5 m MgCl
2
), 66 ìl
NBT (50 mg/ml nitro blue tetrazolium in 70%
dimethylformamide) and 33 ìl BCIP (50 mg/ml
5-bromo-4-chloro-3-indolyl phosphate in 70%
dimethylformamide).
RNA for cDNA libraries and Northern blot
analysis
Y. lipolytica was grown in YP medium contain-
ing 2% glucose or 2% glycerol at 30
C. Cells were
harvested late in the logarithmic phase at an
optical density (OD
600
) of 5·5, frozen in liquid
nitrogen and powdered. Total RNA was iso-
lated by the guanidium thiocyanate method fol-
lowed by ultracentrifugation through a 5·7
CsCl cushion (Chirgwin et al., 1979). Poly(A
+
)
RNA
was
isolated
by
oligo(dT)-cellulose
a
ffinity chromatography (Aviv and Leder,
1972; Sambrook et al., 1989). Double-stranded
cDNA was synthesized as described below. For
Northern blot analysis the poly(A
+
) RNAs
(2·5 ìg/sample) were electrophoresed in 1%
agarose, 2·2
formaldehyde gels (Thomas, 1983)
and blotted to a nylon membrane (Hybond-N,
Amersham Corp.) with 10
SSC as transfer
bu
ffer. PCR-generated copies of the L1·41, L1·45,
L2·7 or L2·17 cDNAs were used as probes.
100 ng of DNA (isolated using Qiagen) of the
respective cDNAs were used as templates with
50 pmol of pYES 2·0 specific forward and reverse
primers, a DNA thermal cycler and 2·5 units of
Taq polymerase (Perkin–Elmer). Thirty cycles
of PCR were performed using a cycle profile of
denaturation at 94
C for 30 s, annealing at 55C
for 30 s, and extension at 72
C for 1 min. The
PCR products were purified with QIAquick PCR
Purification Kit (Qiagen). The probes were
labelled with
32
P (>1
10
9
cpm/ìg), hybridized
to the membrane and washed as described
(Kauppinen et al., 1995). After autoradiography
at
80C, the L1·41 probe was removed from
the membrane according to the manufacturer’s
instructions, and the filter was rehybridized
sequentially to the remaining probes.
Construction of directional Y. lipolytica cDNA
libraries from YP glucose and YP glycerol cultures
Double-stranded cDNA was synthesized from
5 ìg Y. lipolytica poly(A)
+
RNA from each of the
YP glucose and YP glycerol cultures, as described
(Gubler and Ho
ffman, 1983; Sambrook et al.,
1989) except that 1·5 ìg of oligo(dT
18
) NotI primer
(Pharmacia) was used in the first strand reaction.
After synthesis, the cDNA was ligated to non-
palindromic BstXI adaptors (Invitrogen), using a
50-fold molar excess of the adaptors. The adapted
cDNAs were digested with NotI, size fractionated
by agarose gel electrophoresis, ligated into BstXI/
NotI cleaved pYES 2·0 vector (Invitrogen), and the
ligation mixtures were transformed into electro-
competent E. coli DH10B cells (Gibco BRL). The
libraries, consisting of 2
10
6
(L1) and 3
10
5
(L2) clones (glucose- and glycerol-based cultures,
respectively), were stored in 20% glycerol at
80C.
Southern blot analysis
Y. lipolytica total DNA was prepared from
20 ml YP glucose overnight cultures. Cells were
resuspended in 400 ìl 0·9
Sorbitol, 0·1 EDTA
pH 7·5, 14 m
â-mercaptoethanol and incu-
bated for 30 min at 37
C with 100 ìl Novozyme
(2 mg/ml) (Novo Nordisk A/S). Spheroplasts were
resuspended in 400 ìl TE and 5 ìl 10
RNase
A+T added, 90 ìl fresh-made 280 m
EDTA
pH 8·0, 444 m
Tris, and 2·2% SDS, and incu-
bated for 30 min at 65
C. The suspension was
added to 80 ìl 5
potassium acetate, mixed and
cooled on ice for 30 min. The supernatant was
extracted with phenol/chloroform at 65
C and the
DNA was precipitated with ethanol.
The Y. lipolytica total DNA was digested to
completion with BamHI, EcoRI, HindIII or KpnI
(10 ìg/sample), fractionated on a 1% agarose gel,
denatured, and transferred to a nylon filter
(Hybond-N, Amersham) using 10
SSC as trans-
fer bu
ffer (Southern, 1975). PCR copies of the
L1·41, L1·45, L2·7 and L2·17 cDNAs were used
as probes.
32
P-labelling, hybridization, washing,
autoradiography and removal of the probe from
the membrane prior to rehybridization were
carried out as described for Northern blot analysis.
Construction and screening of a Y. lipolytica
genomic library using PCR generated probes
Total DNA was prepared as described for
Southern blot analysis. Partial Sau3AI digested
1271
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

DNA was fractionated on a 1% low melting point
agarose gel. Fragments of 1–2 kb were recovered
from the gel, ligated into the BamHI site of
pSJ1678 and transformed into E. coli SJ2. A
genomic library of >45,000 clones was obtained.
Sau3AI sites were shown to be present internally
in the L1·41 and L2·7 cDNAs (data not shown). In
order to increase the probability of identifying
sequences located upstream of the coding part of
the corresponding genomic sequences, 400 bp PCR
generated copies of the 5
end of the L1·41 and
L2·7 cDNAs were used as probes for screening
of the genomic library. PCR was performed as
described for RNA gel blot analysis. L1·41 cDNA
specific primers: forward 5
-CCTTCTGAGTATA
AGAATC-3
, reverse 5-GATACCAGCCTCGA
ACTC-3
. L2·7 cDNA specific primers: forward
5
-GATCGCAGCTCTCTCCCAC-3, reverse 5-
GACCTTGATGAGAGGGTC-3
.
The Y. lipolytica genomic library was screened
by colony hybridization (Sambrook et al., 1989)
using random-primed (Feinberg and Vogelstein,
1983)
32
P-labelled (>1
10
9
cpm/ìg) PCR prod-
ucts for L1·41 and L2·7 as probes.
32
P-labelling
and hybridization as described (Kauppinen et al.,
1995).
Cloning of PCR generated EF-1á and RPS7
promoter sequences in Y. lipolytica expression
vectors
PCR of the EF-1á and RPS7 genomic sequences
was performed using conditions described for
Northern blot analysis except that the annealing
temperature was raised to 60
C. EF-1á genomic
sequence specific primers: forward 5
-CCATCGA
TAGAGACCGGGTTGGCG-TATTTGTGTCC-
3
, reverse 5-CGCGGATCCTTCGGGTGTGG
AGTTGACAAGG-3
. RPS7 genomic sequence
specific primers: forward 5
-CCATCGATTACCT
GCTACTTG-TCTCAACACC-3
, reverse 5-CGC
GATCCTTGTGTTTGTTGAGTGAAGAAAAG-
ATTTGG-3
. For both promoter sequences the 3
terminal nucleotide was defined to the last nucleo-
tide in the genomic sequence that was not repre-
sented in the corresponding cDNA sequence 5
end. The PCR-generated promoter sequences were
digested with BamHI and ClaI and cloned as
5
–ClaI 3–BamHI fragments in pY3 derivates
) from which the XPR2 promoter
sequence was deleted. The cloning sites were intro-
duced into the promoter sequences by the syn-
thetic oligonucleotides used in the PCR reaction.
The PCR-generated promoter sequences were
confirmed by DNA sequencing.
Nucleotide sequence analysis of the Y. lipolytica
cDNAs and genomic sequences
All nucleotide sequences were determined by the
dideoxy chain-termination method (Sanger et al.,
1977) using 500 ng of Qiagen-purified template
(Qiagen), ABI PRISM
dye terminator cycle
sequencing ready reaction kit with Amplitag
DNA polymerase, FS (Perkin–Elmer) and 5 pmol
of either pYES 2·0 polylinker primers (Invitrogen)
or synthetic oligonucleotide primers. Reactions
were analysed on ABI PRISM 377 according to
manufacturers’ instructions.
Two sets of 100 cDNA 5
tags from subpools
of each Y. lipolytica library were sequenced on
one strand using the pYES 2·0 polylinker primer
(forward). The complete sequence of the selected
L1·41 and L2·17 cDNAs were determined on both
strands using the pYES 2·0 polylinker primers and
synthetic oligonucleotide primers. Sequence com-
parisons were carried out using FASTA searches
(Pearson and Lipman, 1988) on the GenEMBL
database.
Insert DNA from positive clones from the
Y. lipolytica genomic library was subcloned
into pUC19 (Yanisch-Perron et al., 1985) and
sequenced on both strands using synthetic oligo-
nucleotide primers.
Nucleotide sequence accession numbers
The TEF and RPS7 gene promoter sequences
presented in this paper, the TEF and RPS7 gene
cDNA sequences, and the cDNA sequences of the
fungal test genes have been submitted to GenBank
and assigned the following Accession Num-
bers: Genomic sequences: TEF gene promoter,
AF054508; RPS7 gene promoter, AF054509.
cDNA sequences: TEF, AF054510; RPS7,
AF054511; Cellulase I, AF054512; Cellulase II,
A21793; Galactanase I, L34599; Xylanase I,
X76047; Polygalacturonase I, AF054893; Lipase I,
AF054513.
RESULTS AND DISCUSSION
Criteria for evaluation of the alternative yeasts
To examine the potential of the four alternative
yeasts as host organisms for expression cloning, six
test genes donated by three filamentous fungal
1272
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

strains (
) were introduced in autonomously
replicating expression vectors under the control of
strong homologous promoters (
). The yeast
transformants were tested in various rich and
minimal media containing di
fferent carbon sources
in order to maximize the secretion of active en-
zyme (data not shown). The optimal media found
in these preliminary experiments are described
in Materials and Methods. The following were
examined for:
1. The amount of active enzyme secreted. This is
the most important of the listed parameters in
the search for an attractive expression cloning
host organism. A high expression level of active
enzyme will increase the probability of identify-
ing cloned genes by plate screenings. Further,
the e
fficiency of the expression cloning system
would be significantly improved if the organism
used for the initial cloning of new enzyme
genes subsequently was able to express the
gene products in su
fficient amounts to enable
characterization.
2. Glycosylation pattern. This was monitored by
Western blots. This analysis made it possible to
observe posttranslational modifications in the
various hosts compared to the native enzymes.
The blots could also reveal whether the
specific activity of the enzymes was significantly
altered by comparing the amount of enzyme on
the blot with the measured enzyme activity
3. Transformation frequency and plasmid stability.
In the context of expression cloning, the trans-
formation frequency of the host organism
relates to the necessity of screening standard
cDNA libraries. When introduced in the hosts,
allow identification of positives, preliminary
production and, eventually, plasmid rescue.
Initial results demonstrated that variation in the
5
non-translated sequence had a significant effect
on the expression level in all the host systems. The
presence of an SfiI restriction site reduced the
expression level up to threefold (data not shown).
This observation could be explained by the poten-
tial for a loop structure formation in the GC-rich
SfiI sequence. In the attempt to level the e
non-translated sequence in the
various host systems, an SfiI site (N-terminal) and
a NotI site (C-terminal) was introduced in all the
test genes. This enabled swapping of the test genes
between the expression vectors, thus maintain-
ing identical 5
non-translated sequences in the
di
fferent yeasts.
In an experimental setup such as the one
described here, it is di
fficult to compare the differ-
ent systems because of factors such as the relative
strength of the chosen promoters, copy number of
the individual vectors and use of individual growth
media, but the systems are standardized as much
as possible and, in case significant variation in the
capacity of the various hosts was observed we
would be able to select candidates for further
optimization.
Enzyme activity in supernatants
Samples were taken from the cultures three
times during the logarithmic growth phase and
three times during the stationary growth phase.
The substrate degradation capacity present in
the supernatants was measured and related to the
degradation capacity of known amounts of the
corresponding native enzymes as described in
Materials and Methods. The enzyme activity
values presented in
arise from the samples
where the average activity per volume supernatant
was highest. For all transformants, maximum
activity was observed in one of the samples from
the stationary growth phase corresponding to
60–90 h of growth. The enzyme activity values are
presented both as activity per volume of super-
natant (
). In the experiments described, the cells were
grown in conical flasks where a significant strain-
dependent variation in cell density of the cultures
was observed, therefore actvity per cell is a more
accurate measurement for comparative purposes.
Plasmid loss has not been considered in the values
A comparison of the enzyme activities shows
that all the alternative yeast strains secrete active
forms of the test gene products at a significantly
higher level than S. cerevisiae. This result is not
surprising, considering the fact that S. cerevisiae
has been used for thousands of years by mankind
in brewing and baking at conditions where the
ability to secrete substrate degrading enzymes to
the environment has been needless. The activity
data further shows that the expression e
fficiency of
fferent yeasts varies significantly in a gene-
dependent way and is independent of the donor
In general, H. polymorpha, S. pombe and Y.
lipolytica secrete most active enzyme/ml (
Figure
1273
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)
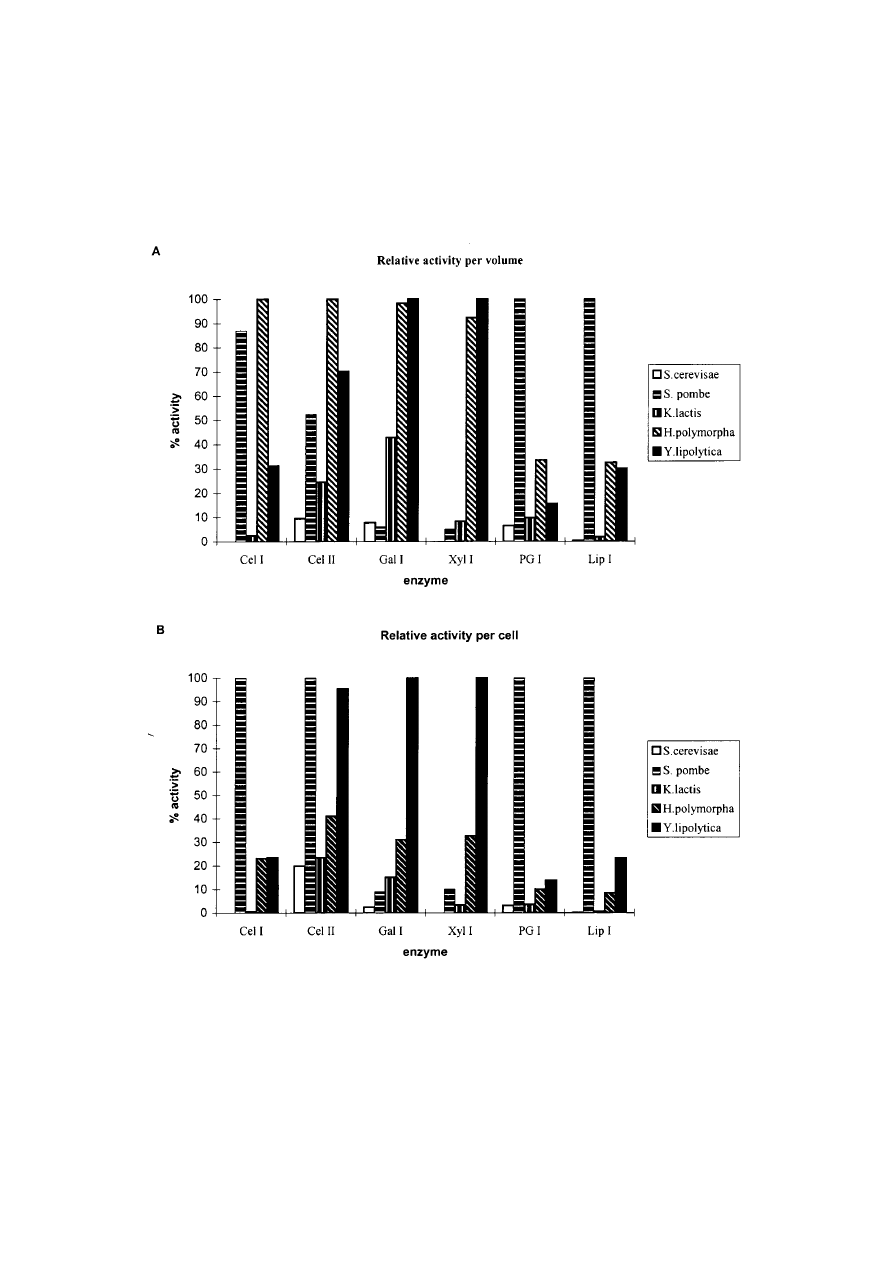
Figure 1. Relative enzyme activity per volume (A), and the actvity divided by the OD
600
value of the respective
yeast culture (B). Samples for measurement of enzyme activity in culture supernatants shown here were taken at the
time of maximal activity per volume. For all transformants this maximum was reached in the stationary growth
phase. The highest activity obtained per volume (A) or per OD
600
(B) for the respective enzymes was set to 100.
Cel I, Cellulase I; Cel II, Cellulase II; Gal I, Galactanase I; Xyl I, Xylanase I; PG I, Polygalacturonase I; Lip I,
Lipase I.
1274
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)
1A
), but in the case of H. polymorpha this is due to
a high cell density (
Western blot analysis, transformation frequency
and plasmid stability
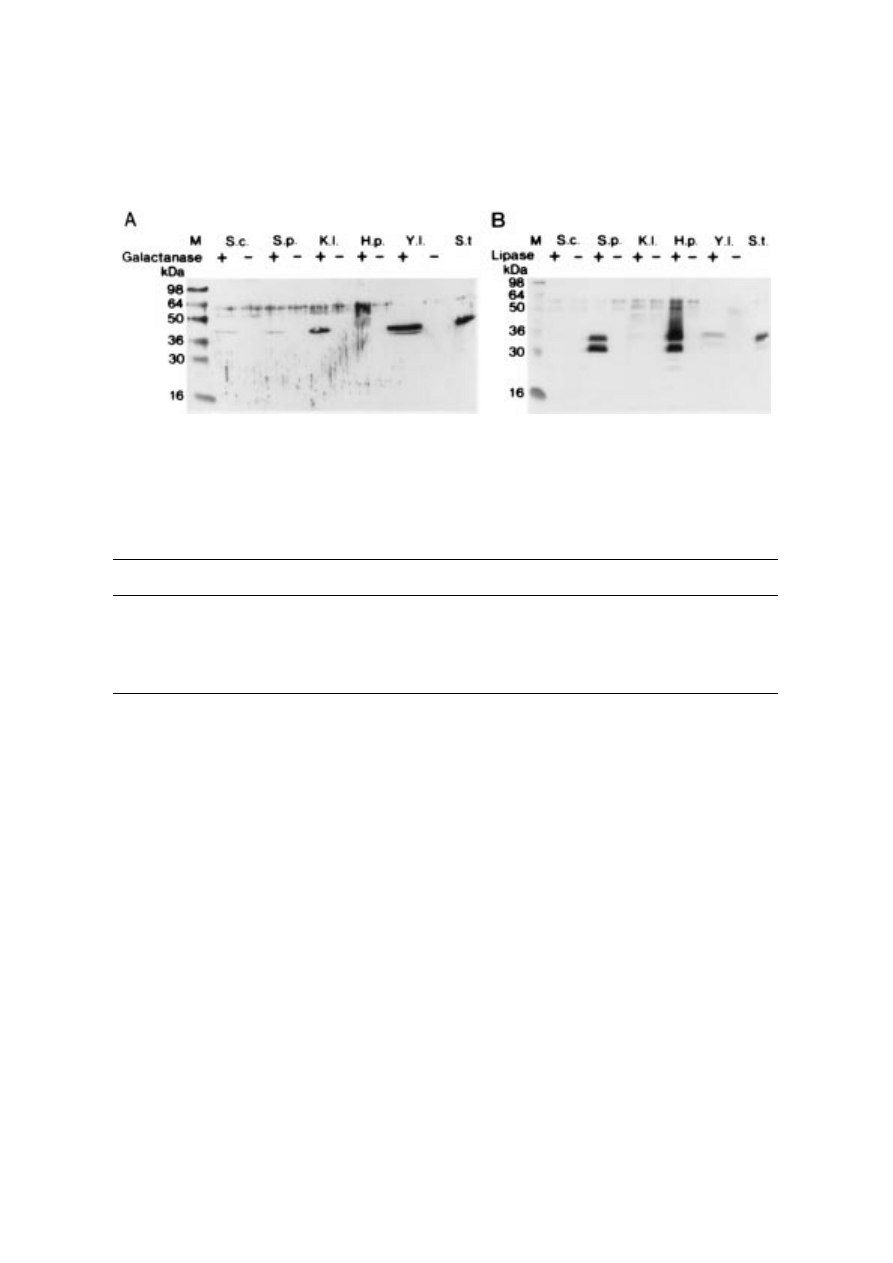
Western blots were carried out to monitor
glycosylation. Examples of Galactanase I (A) and
Lipase I (B) secretion are given in
According to the Western blots, only H. poly-
morpha over-glycosylated significantly. As this
observation has not been reported elsewhere, it
might be a strain-dependent phenomenon. The
over-glycosylation seems to decrease the specific
actvity of Lipase I, as seen when the activity per
volume is compared to the amount of secreted
enzyme (
). Over-glycosylation
does not seem to influence the specific activity of
Galactanase I (
). The Western
blot also reveals that Galactanase I is exposed
to a minor processing when secreted from Y.
lipolytica.
In the preliminary examination of all the alter-
native yeasts, the lowest acceptable transformation
frequency was defined as 1
10
3
cfu/ìg plasmid
DNA. With all the yeasts we could obtain trans-
formation frequencies >1
10
3
, except for S.
pombe.
For all yeast expression systems, enzyme activity
levels increased continually over 72 h in non-
selective medium, indicating that plasmid loss was
not a significant problem.
Evaluation of the alternative host systems
The advantages and disadvantages of the tested
host systems concerning parameters other than the
expression capacity are shown in
Based on the high capacity for secretion of
active forms of the test gene products H. poly-
morpha, S. pombe and Y. lipolytica appear to be
the most promising alternatives to S. cerevisiae as
host organisms for expression cloning. However,
there are also disadvantages, such as over-
Figure 2. Western blots of galactanase (A) and lipase (B) cultures. 25 ìl supernatant aliquots from the samples used for
measurement of enzymatic activity (
) was loaded in each lane. S.c.=S. cerevisiae; S.p.=S. pombe; K.l.=K. lactis;
H.p.=H. polymorpha; Y.l.=Y. lipolytica; M=molecular markers; S.t.=100 ng of the native enzyme; + Indicates the presence of
a test gene in the expression vector;
Indicates the absence of an insert in the expression vector. In the case of lipase I (B), the
upper band is N-glycosylated and the lower is not (Boel and Huge-Jensen, 1989).
Table 3. Advantages and disadvantages of the yeasts concerning expression cloning. Transformation frequency
and growth are illustrated with +–+ + + + +, where + is relative minimum and + + + + + relative maximum.
S. cerevisiae
S. pombe
K. lactis
H. polymorpha
Y. lipolytica
Transformation frequency
+ + + + +
+
+ + + +
+ + +
+ + +
Glycosylation problems
Apparently none Apparently none Apparently none
Up to
20 kDa increase
Apparently none
Plasmid stability
Satisfactory
Satisfactory
Satisfactory
Satisfactory
Satisfactory
Growth
+ + +
+
+ + +
+ + + + +
+ + +
1275
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)
glycosylation of the heterologous products in H.
polymorpha (this was observed for all test gene
products except for Xylanase I) and di
fficulty in
transforming S. pombe.
Complications in the use of Y. lipolytica was
neither related to the character of the secretory
system or handling of the cells, but seemed to be
restricted to the peptone induced XPR2 promoter
employed in the expression vector (see below).
Although the XPR2-based expression system is
unfit in the context of expression cloning, it re-
vealed a high potential of Y. lipolytica as an
alternative to S. cerevisiae, yielding an increase in
expression of the tested gene products of 4·5, 4·8
and 40 times (Polygalacturonase I, Cellulase II
and Galactanase I, respectively) and, in case of
Cellulase I, Xylanase I and Lipase I, an increase of
more than two orders of magnitude.
As the XPR2 promoter revealed a high potential
of Y. lipolytica as an alternative candidate, we
decided to optimize the Y. lipolytica expression
system by identification and integration of more
suitable homologous promoters.
Identification of suitable Y. lipolytica promoters
In the context of the expression cloning method,
optimization of the expression vector, by replace-
ment of the employed XPR2 promoter, was neces-
sary for several reasons: the XPR2 promoter is
only active at pH>6·0 on media lacking preferred
carbon and nitrogen sources and full induction
requires high levels of peptone in the culture
medium (Ogrydziak et al., 1977; Ogrydziak and
Scharf, 1982). The presence of peptone in the
medium complicates product recovery and puri-
fication and hinders the direct screening for
transformants based on LEU2 selection.
In the attempt to identify new strong promoters,
we constructed cDNA libraries from cultures
grown under conditions suitable for use in expres-
sion cloning. Determination of sequence tags
facilitated identification of highly expressed genes
and the isolation of the corresponding promoter
sequences from a genomic Y. lipolytica library.
This strategy was based on the assumption that a
high level of a specific mRNA, under conditions
suitable for expression cloning, reflects the pres-
ence of a strong promoter which is active under
these conditions. cDNA libraries were constructed
from YP-glucose and -glycerol cultures. By
examination of the gene activity in two di
fferent
assimilable media we hoped to identify not only
strong but also inducible (e.g. catabolite-repressed)
promoters.
Initial sequence determination was performed
on 100 clones from each cDNA library in which
300–600 nucleotides of the 5
end of the inserts
were determined. The sequence data from each
library were aligned against each other. In the
following, the cDNA library from the YP-glucose
culture is referred to as L1 and the library from the
YP-glycerol culture as L2. The number following
L1 or L2 refers to a specific clone in the library.
The sequence alignment revealed that six di
fferent
sequences from L1 were represented twice and two
di
fferent sequences were represented as triplets.
Twelve di
fferent sequences from L2 were repre-
sented twice. Alignment of L1 and L2 showed that
several sequences from one library also were rep-
resented in the other. Four sequences were chosen
for further examination: one representing a se-
quence observed as a triplet in L1 and twice in L2
(L1·41), one representing a sequence observed as
a triplet in L1 but not observed in L2 (L1·45),
one representing a sequence observed twice in L1
and L2 (L2·7), and one representing a sequence
observed twice in L2 and not in L1 (L2·17).
The detection of a sequence in two or three
copies in only one of the cDNA libraries could
indicate that di
fferent promoter activity was
present in the YP-glucose and -glycerol media. To
test this, a Northern blot analysis was performed.
Equal intensities of signals using poly(A
+
) RNA
from both glucose and glycerol grown cultures
revealed that these genes were not under control of
a glucose-repressed promoter (data not shown).
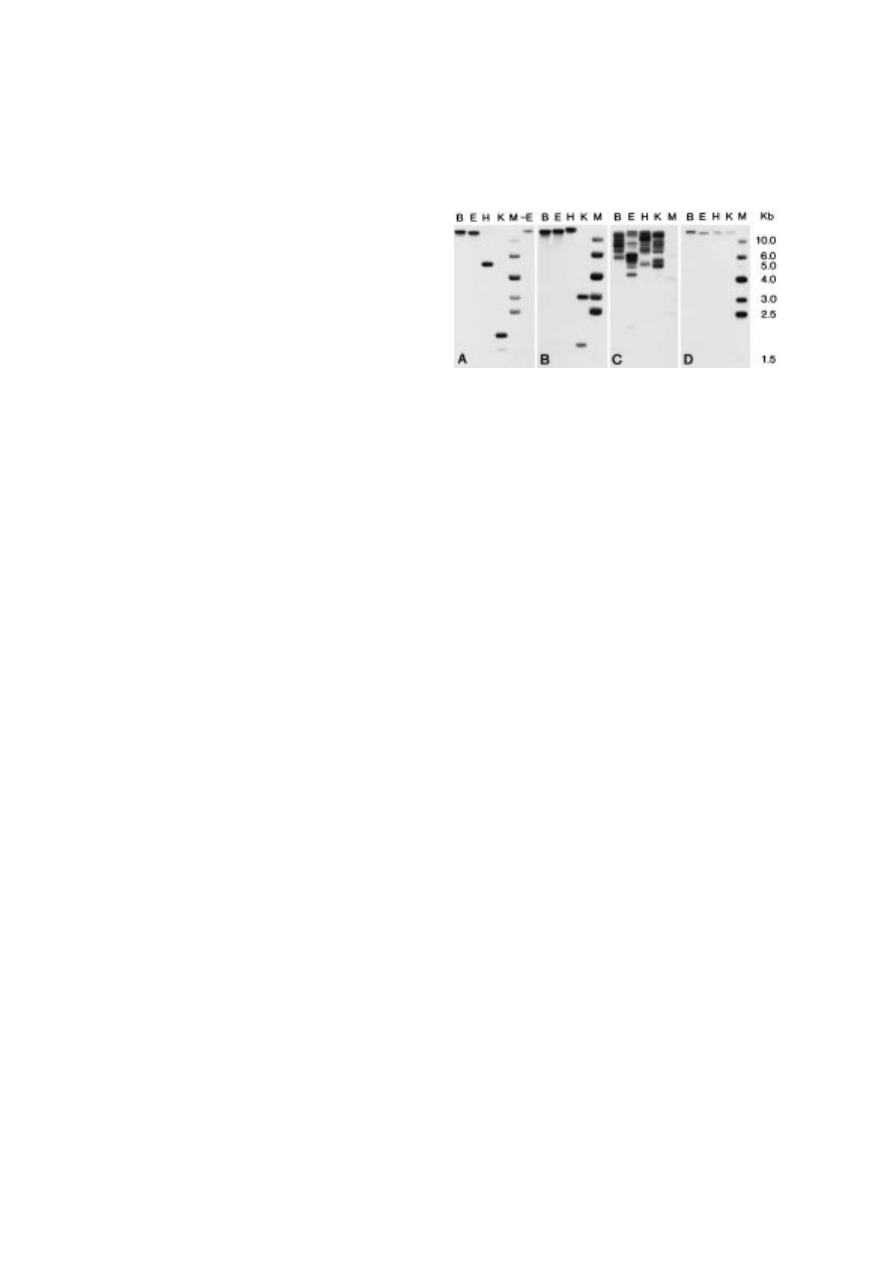
In order to examine whether the high frequency
of the selected cDNAs was due to a high copy
Figure 3. Examination of copy number by Southern blot
analysis. Y. lipolytica total DNA was cut to completion with
BamHI (B), EcoRI (E), HindIII (H) or KpnI (K). M is a marker
and -E is a DNase contamination control in which no enzyme
was present during the incubation. PCR-generated copies of the
selected cDNAs were used as probes: A=L1·41; B=L1·45;
C=L2·7; D=L2·17.
1276
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

number of the corresponding genes, a Southern
blot analysis in combination with a restriction
analysis was carried out (
). Probes based
on the L1·41 (A), L1·45 (B) or L2·17 (D) sequences
only hybridize at one or two areas on the mem-
brane and in the case of two signals, this is due to
the presence of the respective restriction enzyme
recognition sequences in the structural genes. This
strongly indicates that the P
0
1d genome contains
only one copy of each of L1·41, L1·45 and L2·17.
The L2·7-based probe hybridizes at several distinct
areas on the membrane. Digest of a PCR copy of
the L2·7 sequence with the employed enzymes did
not reveal an internal presence of these sites (data
not shown). This shows that the L2·7 sequence
is present in several copies of the P
0
1d genome.
For this reason no attempt was made to
identify and test the promoter matching the L2·7
sequence.
Cloning of Y. lipolytica TEF and RPS7 promoter
sequences
To identify the promoters controlling the
strongly expressed transcripts, a Y. lipolytica
genomic library was screened using the first 400 bp
of the genes as probes. Colony hybridization
resulted in the isolation of two genomic sequences
matching the L1·41 and L2·17 cDNAs, respect-
ively. The 1·5 kb L1·41 and 0·9 kb L2·17 cDNAs
were sequenced to completion and aligned by
FastA searches (Pearson and Lipman, 1988) to the
GenEMBL database. The L1·41 cDNA sequence
showed significant homology to the translation
elongation factor-1á (TEF) gene of various
sources, e.g. Arxula adeninivorans, Neurospora
crassa and Saccharomyces cerevisiae. GAP align-
ments (Needleman and Wunch, 1970) to the TEF
gene sequences of the yeasts A. adeninivorans
(accession no. Z47379) and S. cerevisiae (accession
no. X00779) showed 83·8% and 76·4% identity,
respectively. The L2·17 cDNA sequence showed
significant homology to the ribosomal protein S7
(RPS7) gene of e.g. S. cerevisiae and the corre-
sponding RPS4 gene of e.g. Drosophila mela-
nogaster and Homo sapiens. GAP alignments
showed 69·2% identity to the RPS4 gene of D.
melanogaster (accession no. D16257) and 68·5%
identity to exon 1 and 2 of the S. cerevisiae
(accession no. M64293) RPS7 gene. Both of the
identified genes encode proteins involved in trans-
lation. The elongation factor-1á plays an essential
role in protein synthesis in eukaryotic cells by
binding the amino-acyl tRNA to the ribosomes in
exchange for the hydrolysis of GTP. The ribo-
somal protein S7 is the largest protein of the 40 S
subunit and is essential for growth (Synetos et al.,
1992). Identification of the TEF gene by the
present approach is not that surprising, as its
product is an exceptionally abundant protein com-
prising 3–10% of the soluble protein in most cells
(Cavallius et al., 1993). Ribosomal proteins are
also abundant, each representing 0·1–0·5% of the
total cellular protein, and their mRNA are corre-
spondingly abundant (reviewed by Warner, 1989).
As both genes were found to be present in only one
copy on the genome, we expected the matching
promoters, especially the TEF gene promoter, to
be strong. This has been shown to be the case for
the TEF gene promoter of several other organisms
(Schirmaier and Philippsen, 1984; Axelos et al.,
1989; Kim et al., 1990; Ursin et al., 1991).
The L1·41 related genomic sequence was deter-
mined and alignment with the matching cDNA
revealed a 366 bp sequence in the genomic DNA
located upstream of the 5
end of the cDNA
). Similarly, a 758 bp genomic DNA
sequence was present upstream of the matching
L2·17 cDNA 5
end (
). Visual inspection
of the putative promoter sequences indicates
the presence of the cis-acting regulatory element
UAS
rpg
, which is found in the promoter region of
several genes encoding highly expressed ribosomal
and house-keeping genes (Leer et al., 1985; Mager,
1988). The 3
terminal nucleotide of the new
promoter sequences cloned in expression vectors
(see below) was defined to be equal to the last
nucleotide in the genomic sequence that was not
represented in the corresponding cDNA.
Examination of the e
ffect of the novel
Y. lipolytica promoters
In order to test the e
ffect of the Y. lipolytica
TEF- and RPS7 gene promoters, PCR generated
copies were cloned into pY3 derivates from which
the XPR2 promoter was removed and the hetero-
logous cellulase II or xylanase I genes (
were present as reporters. The reporter gene
expression level of Y. lipolytica transformed with
these constructs was compared to the correspond-
ing expression level in cells transformed with the
original XPR2 containing pY3. Transformants
containing the new promoters were grown in
media in which glucose was present as the carbo-
hydrate source.
1277
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

To examine the potential of the new promoters
as screening tools, the transformants were inocu-
lated on to substrate-containing growth plates
). TEF and RPS7 gene promoter trans-
formants were grown on selective inducing SC-
leu+glucose plates, whereas XPR2 transformants
were grown on non-selective, inducing peptone-
containing plates (see Materials and Methods).
The substrate degradation e
ffected by the Y. lipo-
lytica transformants was compared with the
corresponding degradation caused by S. cerevisiae
transformants inoculated on to selective inducing
SC-ura+galactose plates. The plate assay does not
reflect the expression level of the individual trans-
formants as the amount of cells varies but it
mimics an expression cloning screening event.
shows that the TEF gene promoter
very e
ffectively causes substrate degradation
(A, pY5TACII; D, pY5TAXI) in both the HE-
cellulose and Birch-xylan enzyme assays. As a
screening tool the TEF gene promoter appears
more e
ffective than the XPR2 promoter (B and E)
as well as the original expression cloning system
based on S. cerevisiae (C and F). Furthermore, the
plate assay demonstrates that both the new pro-
moters, in contrast to the XPR2 promoter,
are active under selective conditions and thereby
enable the use of Y. lipolytica as a host for direct
screening of cDNA libraries.
The e
ffect of the new promoters was also com-
pared to the e
ffect of the XPR2 promoter in a
quantitative analysis by measuring the enzyme
activity in liquid culture supernatants. As in the
plate assay, Y. lipolytica transformed with the new
promoter constructs were grown in selective induc-
ing media, whereas the XPR2 promoter transform-
ants were grown in non-selective inducing medium.
The relative enzyme activity values shown in
Figure 4. (A) Nucleotide sequence of the EF-1á promoter. The positions are related to the A
in the ATG start codon (bold) defined as +1. The putative UAS
rpg
boxes HOMOL1 (positions
191 to 180) and RPG (179 to 168) and the T-rich sequence are underlined. The
putative TATA box (
111 to 106) and a pyrimidine-rich sequence (85 to 58) are
double underlined. The putative transcription initiation site (
56 to 53) is written in bold.
Nucleotides located from position
40 and downstream were also present in the cDNA
sequence. (B) Nucleotide sequence of the RPS7 promoter. The positions are related to the A in
the ATG start codon (bold) defined as +1. The putative UAS
rpg
boxes HOMOL1 (positions
273 to 262) and RPG (247 to 236) and the T-rich sequence (present on the opposite
strand) are underlined. Putative TATA boxes (
201 to 190), a TATA-like sequence (46
to
41) and transcription initiation consensus sequences (85, 55, 15 and 13) are
double underlined. Nucleotides located from position
2 were also present in the cDNA
sequence.
1278
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

have not been corrected for the di
fference
in percentage loss of plasmid. As the maximum cell
density was approximately the same in all cultures,
only the relative activity per volume is illustrated.
As in the initial study of the alternative host
systems, the activity values are from samples in the
stationary growth phase where the activity per
volume supernatant was highest. It should be
noted that the samples from the TEF and RPS7
gene promoter cultures do not necessarily repre-
sent the highest activity level possible, as no stag-
nation in the activity level was observed among the
stationary growth phase samples taken (data not
shown). The samples from the TEF and RPS7
gene promoter cultures presented in
were
taken after 80 h of cultivation. The continued
increase in enzyme activity is not surprising con-
sidering the need of the TEF and RPS7 gene
products in the maintenance of a cell. Ro¨sel and
Kunze (1995) compared the TEF gene mRNA
levels at di
fferent times during yeast growth of the
dimorphic yeast Arxula adeninivorans, in which the
TEF gene also seems to be present in only one
copy on the genome, in a Northern blot analysis.
As in the present growth experiment, glucose was
used as the carbon source. The transcript level
decreased when the culture entered the stationary
growth phase but then stabilized, and no di
fference
was observed among the last-taken 60 and 75 h
samples.
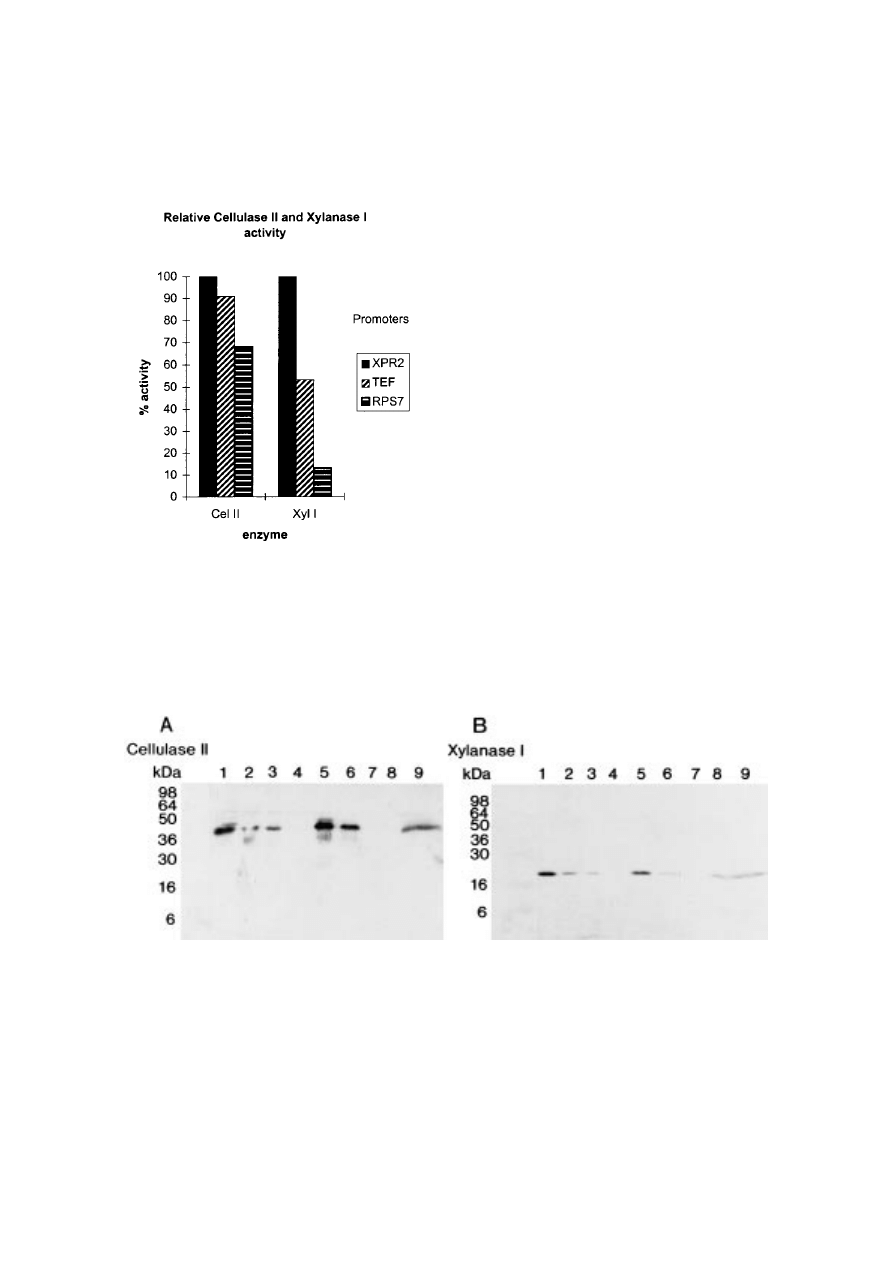
In case of the reporter genes tested here the TEF
gene promoter appears as the most striking of the
novel promoters. In the Cellulase II samples com-
pared, the activity level of the enzyme under
control of the TEF gene promoter almost equals
that of the XPR2 promoter. The e
ffect of the RPS7
gene promoter is roughly 30% less than the e
ffects
of the TEF gene promoter. Interestingly, use of
the xylanase I reporter gene seems to a
ffect the
tested promoters very di
fferently. As observed for
Figure 5. The e
ffect of the Y. lipolytica EF-1á (pY5TACII/XI) and RPS7 (pY5RBCII/XI) promoters (A and D), as indicated
by use of plate assays. The novel promoters were introduced in pY3 derivatives in which the XPR2 promoter was absent and with
cellulase II or xylanase I as reporter genes. Positive cellulase II (A) and xylanase I (D) transformants were reinoculated on to
SC-leu plates containing 2% glucose, 0·1% AZCL HE-cellulose (A) or AZCL Birch-xylan (D) substrate. CV=control vectors, in
which no promoter sequence was present. Y. lipolytica XPR2 (pY3CII/XI) promoter-based expression of Cellulase II (B) or
Xylanase I (E). The cellulase II or xylanase I test genes were cloned in pY3 and positive transformants were reinoculated on to
inducing non-selective plates (0·2% yeast extract, 0·1% glucose and 10% proteose peptone in 50 m
NaHPO
4
, pH 6·8) containing
the relevant AZCL substrate. Cellulase II (C) and xylanase I (F) expression from the original expression cloning host S.
cerevisiae. The test genes were cloned in pYES 2·0 downstream of the Gal1 promoter and positive transformants were restriked
on SC-ura plates containing 2% galactose and the relevant AZCL substrate. Cellulase II transformants (A, B and C) were
incubated at 30
C for 48 h. Xylanase I transformants (D, E and F) were incubated at 30C for 24 h.
1279
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)
Cellulase II expression, the enzyme activity is
higher in the presence of the TEF gene promoter
than in the presence of the RPS7 gene promoter,
but in the case of Xylanase I activity the TEF gene
promoter appears four times more e
ffective.
Whereas the e
ffect of the TEF gene promoter in the
case of Cellulase II activity almost equals the e
ffect
of the XPR2 promoter, it only corresponds to
about 50% of the XPR2 promoter e
ffect in the case
of Xylanase I activity. The Western blot analysis
) shows a decrease in the amount of
secreted product as reflected in the enzyme
activities shown in
The tests of the new promoters on substrate-
containing plates (
) indicated that the new
promoters were significantly more e
ffective than
the XPR2 promoter. The activity assay on the
supernatants (
) showed that this was not
the case when the cells were grown in liquid
medium. A possible explanation of these conflict-
ing observations could be that the environmental
demands for full induction of the XPR2 promoter
(e.g. pH 6·8) are not maintained in the growth
plates—this would also explain why pYES 2·0 in
S. cerevisiae appears as a more e
ffective ex-
pression system than XPR2-based expression in
Figure 6. Maximum Cellulase II and Xylanase I activity in
supernatants from Y. lipolytica cultures in the presence of the
XPR2, EF-1á or RPS7 promoters. The highest activity ob-
tained for the respective enzymes was set to 100%. In case of
Cellulase II expression, 100% correspond to the activity of
7·6 mg/l native enzyme. 100% Xylanase I activity corresponds
to the activity of 1·9 mg/l native enzyme.
Figure 7. Western blot of Y. lipolytica Cellulase II (A) and Xylanase I (B) cultures based on the new promoters and XPR2.
(A) Lane 1=0·3 ìg, 2=0·15 ìg, 3=0·075 ìg native Cellulase II. Lanes 4, 7 and 8 are empty. Lane 5 is loaded with 25 ìl
supernatant from TEF gene promoter transformants, which correspond to the activity of 0·19 ìg native enzyme. Lane 6 is
loaded with 25 ìl supernatant from RPS7 gene promoter transformants, which correspond to the activity of 0·1 ìg native
enzyme. Lane 9 is loaded with 25 ìl supernatant from XPR2 transformants, which correspond to the activity of 0·19 ìg native
enzyme. (B) Lane 1=0·0375 ìg, 2=0·0075 ìg, 3=0·00375 ìg native Xylanase I. Lanes 4, 7 and 8 are empty. Lane 5 is loaded
with 25 ìl supernatant from TEF gene promoter transformants, which corresponds to the activity of 0·0123 ìg native enzyme.
Lane 6 is loaded with 25 ìl supernatant from RPS7 gene promoter transformants, which correspond to the activity of
0·0048 ìg native enzyme. Lane 9 is loaded with 25 ìl supernatant from XPR2 transformants, which corresponds to the activity
of 0·0317 ìg native enzyme.
1280
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

Y. lipolytica in the case of the plate assay (
) and not in the liquid assay.
The initial examination of Hansenula poly-
morpha, Kluyveromyces lactis, Schizosaccharo-
myces pombe and Yarrowia lipolytica as possible
alternative host organisms in expression cloning
showed that all of them are able to secrete active
forms of the test gene products at a higher amount
than the original host organism S. cerevisiae. The
expression capacity of the various host systems
varied significantly in a gene-dependent manner.
These results show that S. cerevisiae is certainly
not the optimal choice for a host organism and
that improvement of the expression cloning
method probably implies handling of more than
one host organism.
Following the comparative yeast study, we
focused on optimization of the attractive alterna-
tive host Y. lipolytica by replacement of the
initially employed XPR2 promoter. Based on the
present results, the TEF gene promoter seems to be
the most promising of the new Y. lipolytica pro-
moters. As the TEF gene promoter of various
other organisms has proved to be strong, it ap-
pears as an attractive tool for various purposes,
e.g. homologous or heterologous gene expression.
So far, only a few groups have tested the TEF gene
promoter in vivo (e.g. Axelos et al., 1989; Nakari-
Seta¨la¨ and Penttila¨, 1995) and these results indicate
that the strength of the promoter depends on
the origin. Our comparative examination of the
Y. lipolytica TEF gene- and XPR2 promoters
strongly indicates that, in the case of Y. lipolytica,
the TEF gene promoter is a very e
ffective tool for
expression of heterologous proteins.
In the present work we have demonstrated that
one of the most attractive of the examined alter-
native yeasts, Y. lipolytica, by use of an XPR2
promoter-based expression vector, expresses active
forms of the six tested gene products at significant
higher amounts than S. cerevisiae—ranging from
4·5 times to more than two orders of magnitude.
Subsequently, we have cloned two strong pro-
moters from Y. lipolytica, of which especially the
TEF gene promoter seems almost as e
fficient as the
XPR2 promoter but at conditions more suitable
for use in expression cloning. Use of Y. lipolytica
as the host organism in a TEF gene promoter-
based expression system will definitely increase the
number of positive cDNA clones to be detected
and allow production of some of the cloned
genes in su
fficient amounts to enable initial
characterization.
ACKNOWLEDGEMENTS
We thank Dr Fiona Du
ffner and Dr Sakari
Kauppinen for useful discussions and Heidi
Heinsøe, Jannie Steinvig, Lars A. Petersen and
Mari-Ann Allerslev for skilful technical assistance.
REFERENCES
Axelos, M., Bardet, C., Liboz, T., Thai, A. L. V., Curie,
C. and Lescure, B. (1989). The gene family encoding
the Arabidopsis thaliana translation elongation fac-
tor EF-1á: Molecular cloning, characterization and
expression. Mol. Gen. Genet. 219, 106–112.
Aviv, H. and Leder, P. (1972). Purification of bio-
logically active globin messenger RNA by chroma-
tography on oligothymidylic acid-cellulose. Proc. Natl
Acad. Sci. USA 69, 1408–1412.
Becker, D. M. and Guarente, L. (1991). High-e
fficiency
transformation of yeast by electroporation. Methods
Enzymol. 194, 182–187.
Boel, E. and Huge-Jensen, I. B. (1989). Recombinant
Humicula lipase and process for the production of
recombinant humicula lipases. EP0305216A1.
Bro¨ker, M. and Ba¨uml, O. (1989). New expression
vectors for the fission yeast Schizosaccharomyces
pombe. FEBS Lett. 248, 105–110.
Bro¨ker, M. (1987). Transformation of intact Schizo-
saccharomyces pombe cells with plasmid DNA. Bio-
Techniques 5, 516–518.
Buckholz, R. G. and Gleeson, M. A. G. (1991). Yeast
systems for the commercial production of heterolo-
gous proteins. Bio/Technology 9, 1067–1072.
Cavallius, J., Zoll, W., Chakraburtty, K. and Merrick,
W. C. (1993). Characterization of yeast EF-1á:
non-conservation of post-translational modifications.
Biochim. Biophys. Acta 1163, 75–80.
Chirgwin, J. M., Przybyla, A. E., MacDonald, R. J. and
Rutter, W. J. (1979). Isolation of biologically active
ribonucleic acid from sources enriched in ribo-
nuclease. Biochemistry 18, 5294–5299.
Christgau, S., Kauppinen, S., Vind, J., Kofod, L. V. and
Dalbøge, H. (1994). Expression cloning, purification
and characterization of a â-1,4 mannanase from As-
pergillus aculatus. Biochem. Mol. Biol. Int. 33, 917–925.
Christgau, S., Sandal, T., Kofod, L. V. and Dalbøge, H.
(1995). Expression cloning, purification and charac-
terization of a â-1,4-galactanase from Aspergillus
aculatus. Curr. Genet. 27, 135–141.
Christgau, S., Kofod, L. V., Halkier, T., Andersen,
L. N., Hockauf, M., Do¨rreich, K., Dalbøge, H. and
Kauppinen, S. (1996). Pectin methyl esterase from
Aspergillus aculeatus: expression cloning in yeast and
characterization of the recombinant enzyme. Biochem.
J. 319, 705–712.
Dalbøge, H. and Heldt-Hansen, H. P. (1994). A novel
method for e
fficient expression cloning of fungal
enzyme genes. Mol. Gen. Genet. 243, 253–260.
1281
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

Draborg, H., Kauppinen, S., Dalbøge, H. and
Christgau, S. (1995). Molecular cloning and expres-
sion in S. cerevisiae of two exochitinases from
Trichoderma harzianum. Biochem. Mol. Biol. Int. 36,
781–791.
Draborg, H., Christgau, S., Halkier, T., Rasmussen, G.,
Dalbøge, H. and Kauppinen, S. (1996). Secretion of
an enzymatically active Trichoderma harzianum endo-
chitinase by Saccharomyces cerevisiae. Curr. Genet.
29, 404–409.
Feinberg, A. P. and Vogelstein, B. (1983). A technique
for radiolabeling DNA restriction endonuclease frag-
ments to high specific activity. Anal. Biochem. 132,
6–13.
Fleer, R., Yeh, P., Amellal, N., Maury, I., Fournier, A.,
Bacchetta, F., Baduel, P., Jung, G., L’Hoˆte, H.,
Becquart, J., Fukuhara, H. and Mayaux, J. F. (1991).
Stable multicopy vectors for high-level secretion of
recombinant human serum albumin by Kluyveromyces
yeasts. Bio/Technology 9, 968–975.
Fournier, P., Guyaneux, L., Chasles, M. and Gaillardin,
C. (1991). Scarcity of ars sequences isolated in a
morphogenesis mutant of the yeast Yarrowia lipo-
lytica. Yeast 7, 25–36.
Gellissen, G. and Hollenberg, C. P. (1997). Applications
of yeasts in gene expression studies: a comparison
of Saccharomyces cerevisiae, Hansenula polymorpha
and Kluyveromyces lactis—a review. Gene 190, 87–
97.
Gleeson, M. A., Ortori, G. S. and Sudbery, P. E.
(1986). Transformation of the methylotrophic yeast
Hansenula polymorpha. J. Gen. Microbiol. 132, 3459–
3465.
Gubler, U. and Ho
ffman, B. J. (1983). A simple and very
e
ffective method for generating cDNA libraries. Gene
25, 263–269.
Kauppinen, S., Christgau, S., Kofod, L. V., Halkier, T.,
Do¨rreich, K. and Dalbøge, H. (1995). Molecular
cloning and characterization of a rhamnogalacturo-
nan acetylesterase from Aspergillus aculeatus. J. Biol.
Chem. 270, 27,172–27,178.
Kim, D. W., Uetsuki, T., Kaziro, Y., Yamaguchi, N.
and Sugano, S. (1990). Use of the human elongation
factor 1á promoter as a versatile and e
fficient expres-
sion system. Gene 91, 217–223.
Kofod, L. V., Kauppinen, S., Christgau, S., Andersen,
L. N., Heldt-Hansen, H. P., Do¨rreich, K. and
Dalbøge, H. (1994). Cloning and characterization of
two structurally and functionally divergent rham-
nogalacturonases from Aspergillus aculatus. J. Biol.
Chem. 269, 29,182–29,189.
Kyhse-Andersen, J. (1984). Electroblotting of multiple
gels: a simple apparatus without bu
ffer tank for rapid
transfer of proteins from polyacrylamide to nitro-
cellulose. J. Biochem. Biophys. Meth. 10, 203–209.
Laemmli, U. K. (1970). Cleavage of structural proteins
during the assembly of the head of bacteriophage T4.
Nature 227, 680–685.
Leer, R. J., Van Raamsdonk-Duin, M. M. C., Mager,
W. H. and Planta, R. J. (1985). Conserved sequences
upstream of yeast ribosomal protein genes. Curr.
Genet. 9, 273–277.
Mager, W. H. (1988). Control of ribosomal protein gene
expression. Biochim. Biophys. Acta 949, 1–15.
Meissner, P. S., Sisk, W. P. and Berman, M. L. (1987).
Bacteriophage ë cloning system for the construction
of directional cDNA libraries. Proc. Natl Acad. Sci.
USA 84, 4171–4176.
Nakari-Seta¨la¨, T. and Penttila¨, M. (1995). Production of
Trichoderma reesei cellulases on glucose containing
media. Appl. Envir. Microbiol. 61, 3650–3655.
Needleman, S. B. and Wunsch, C. D. (1970). A general
method applicable to the search for similarities in the
amino acid sequence of two proteins. J. Mol. Biol. 48,
443–453.
Nicaud, J. M., Fabre, E., Beckerich, J. M., Fournier, P.
and Gaillardin, C. (1989). Cloning, sequencing and
amplification of the alkaline extracellular protease
(XPR2) gene of the yeast Yarrowia lipolytica. J.
Biotechnol. 12, 285–298.
Ogrydziak, D. M., Demain, A. L. and Tannenbaum,
S. R. (1977). Regulation of extracellular protease
production in Candida lipolytica. Biochim. Biophys.
Acta 497, 525–538.
Ogrydziak, D. M. and Scharf, S. J. (1982). Alkaline
extracellular protease produced by Saccharomyces
lipolytica CX161-1B. J. Gen. Microbiol. 128, 1225–
1234.
Pearson, W. R. and Lipman, D. J. (1988). Improved
tools for biological sequence analysis. Proc. Natl
Acad. Sci. USA 85, 2444–2448.
Raleigh, E. A., Murray, N. E., Revel, H., Blumenthal,
R. M., Westaway, D., Reith, A. D., Rigby, P. W. J.,
Elhai, J. and Hanahan, D. (1988). MrcA and McrB
restriction phenotypes of some E. coli strains and
implications for gene cloning. Nucleic Acids Res. 16,
1563–1575.
Ro¨sel, H. and Kunze, G. (1995). Cloning and charac-
terization of a TEF gene for elongation factor 1á
from the yeast Arxula adeninivorans. Curr. Genet. 28,
360–366.
Sambrook, J., Fritsch, E. F. and Maniatis, T. (1989).
Molecular Cloning: A Laboratory Manual, 2nd edn.
Cold Spring Harbor Laboratory Press, New York.
Sanger, F., Nicklen, S. and Coulson, A. R. (1977). DNA
sequencing with chain-terminating inhibitors. Proc.
Natl Acad. Sci. USA 74, 5463–5467.
Schirmaier, F. and Philippsen, P. (1984). Identification
of two genes coding for the translation elongation
factor EF-1á of S. cerevisiae. EMBO J. 3, 3311–3315.
Sherman, F. (1991). Getting started with yeast. Methods
Enzymol. 194, 3–21.
Southern, E. M. (1975). Detection of specific sequences
among DNA fragments separated by gel electro-
phoresis. J. Mol. Biol. 98, 503–517.
1282
. ¨
.
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)

Steiner, S. and Philippsen, P. (1994). Sequence and
promoter analysis of the highly expressed TEF gene of
the filamentous fungus Ashbya gossypii. Mol. Gen.
Genet. 242, 263–271.
Synetos, D., Dabeva, M. D. and Warners, J. R. (1992).
The yeast ribosomal protein S7 and its genes. J. Biol.
Chem. 267, 3008–3013.
Thomas, P. S. (1983). Hybridization of denatured RNA
transferred or dotted to nitrocellulose paper. Methods
Enzymol. 100, 255–266.
Ursin, V. M., Irvine, J. M., Hiatt, W. K. and
Shewmaker, C. K. (1991). Developmental analysis of
elongation-factor-1á expression in transgenic tobacco.
Plant Cell 3, 538–591.
Vernis, L., Abbas, A., Chasles, M., Gaillardin, C. M.,
Brun, C., Huberman, J. A. and Fournier, P. (1997).
An origin of replication and a centromere are both
needed to establish a replicative plasmid in the yeast
Yarrowia lipolytica. Mol. Cell. Biol. 17, 1995–2004.
Warner, J. R. (1989). Synthesis of ribosomes in Sac-
charomyces cerevisiae. Microbiol. Rev. 53, 256–271.
Yanisch-Perron, C., Vieira, J. and Messing, J. (1985).
Improved M13 phage cloning vectors and host
strains: nucleotide sequences of the M13mp18 and
pUC19 vectors. Gene 33, 103–119.
1283
1998 John Wiley & Sons, Ltd.
Yeast 14, 1267–1283 (1998)
Document Outline
- Comparison of Expression Systems in the Yeasts Saccharomyces cerevisiae, Hansenula polymorpha, Klyveromyces lactis, Schizosaccharomyces pombe and Yarrowia lipolytica. Cloning of Two Novel Promoters from Yarrowia lipolytica
- INTRODUCTION
- MATERIALS AND METHODS
- Strains, media and growth conditions
- Plasmids, test genes and transformation procedures
- Measurement of enzymatic activity
- Electrophoresis and Western blotting
- RNA for cDNA libraries and Northern blot analysis
- Construction of directional Y. lipolytica cDNA libraries from YP glucose and YP glycerol cultures
- Southern blot analysis
- Construction and screening of a Y. lipolytica genomic library using PCR generated probes
- Cloning of PCR generated EF-1alpha and RPS7 promoter sequences in Y. lipolytica expression vectors
- Nucleotide sequence analysis of the Y. lipolytica cDNAs and genomic sequences
- Nucleotide sequence accession numbers
- RESULTS AND DISCUSSION
- Criteria for evaluation of the alternative yeasts
- Enzyme activity in supernatants
- Western blot analysis, transformation frequency and plasmid stability
- Evaluation of the alternative host systems
- Identification of suitable Y. lipolytica promoters
- Cloning of Y. lipolytica TEF and RPS7 promoter sequences
- Examination of the effect of the novel Y. lipolytica promoters
- ACKNOWLEDGEMENTS
- REFERENCES
Wyszukiwarka
Podobne podstrony:
Comparison of Different Fibers in the Solid Phase Microextra
FIDE Trainers Surveys 2013 07 02, Uwe Boensch The system of trainer education in the German Chess F
Solube expression of recombinant proteins in the cytoplasma of E coli
Język angielski Political System In The UK
Antigone Analysis of Greek Ideals in the Play
Low Temperature Differential Stirling Engines(Lots Of Good References In The End)Bushendorf
Formation of heartwood substances in the stemwood of Robinia
Illiad, The Role of Greek Gods in the Novel
A Critique of Socrates Guilt in the Apology
Hippolytus Role of Greek Gods in the Euripedes' Play
Byrd, emergence of village life in the near east
Chizzola GC analysis of essential oils in the rumen fluid after incubation of Thuja orientalis tw
The Grass Is Always Greener the Future of Legal Pot in the US
Erosion of Secular Spaces in the UK
Alta J LaDage Occult Psychology, A Comparison of Jungian Psychology and the Modern Qabalah
The political system in the UK loskominos
Chizzola GC analysis of essential oils in the rumen fluid after incubation of Thuja orientalis tw
więcej podobnych podstron