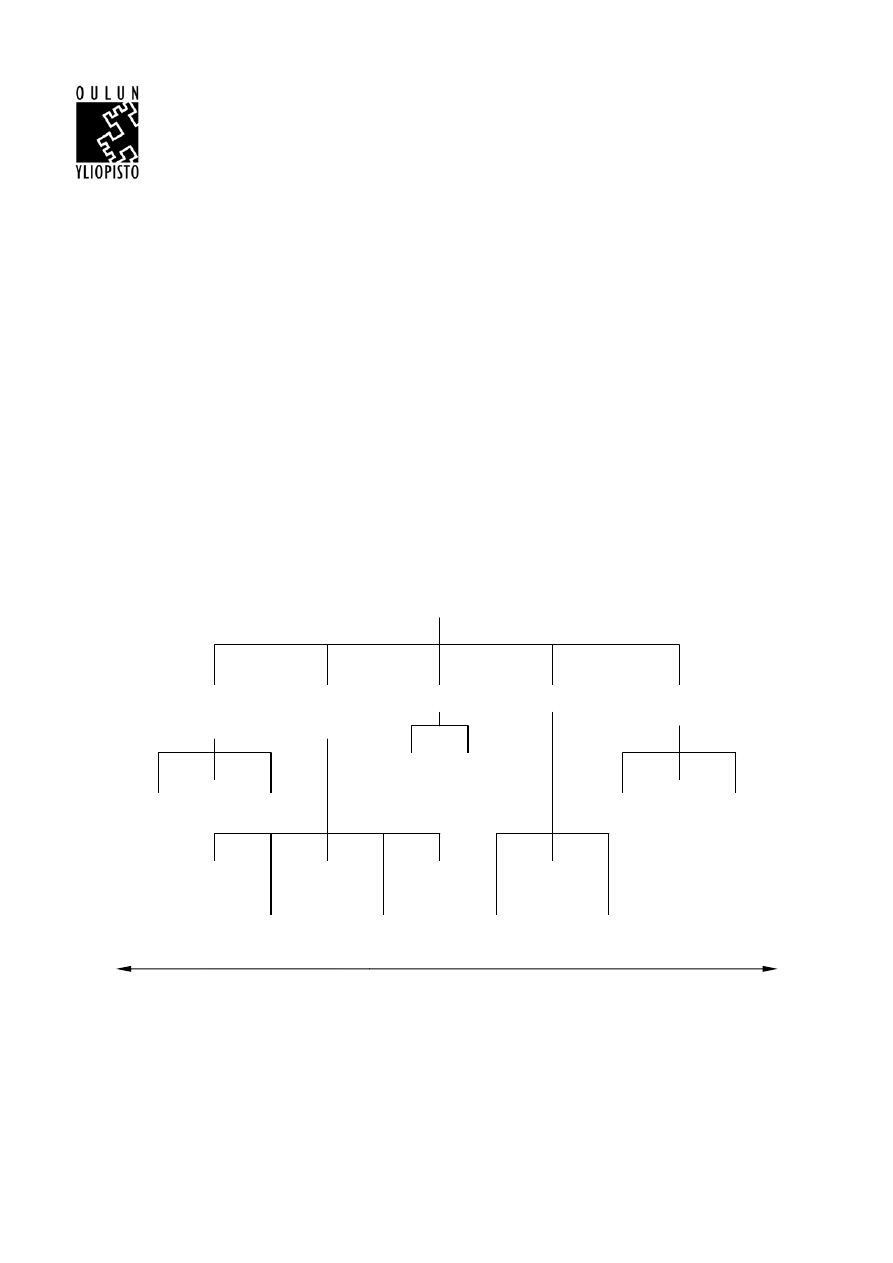
Department of Process and Environmental Engineering
28.4.2005
Mass and Heat Transfer Process Laboratory
Jenni Ylä-Mella
480360S Environmental Catalysis (3 cr.)
RECYCLING OF POLYMERS
Plastics have become common materials of our everyday lives, and many of their properties, such as
durability, versatility and light-weight, can be a significant factor in achieving sustainable
development. However, plastic applications also contribute to the growing amounts of solid waste
generated, as plastic products are often used only once before disposal. The disposal problem is not
simply technical, but it also has social, economic and even political aspects. This is the reason why
several different methods have been explored and applied for solving the problems associated with
polymer waste handling and disposal. (Strong 2000) The alternatives of practical techniques for solid
waste management are shown in Figure 1.
Clean
technology
redesign
Waste source
elimination or
reduction
Reprocessing
External recycling
Disposal
methods
Process
redesign
Alternative
input
material
Product
redesign
Reworking Material
recovery
Awareness
training
monitoring
Process
modelling and
optimization
Customer
co-operation
Least profitable
Most profitable
Landfill
etc.
Incineration
Treatment
Incineration
By-product
Recycling /
Recovery
Input material
changes
Good operating practices,
maintenance and good
housekeeping
WASTE MANAGEMENT TECHNIQUES
Figure 1 Practical techniques for waste management (Phillips 2000).
Even though external recycling is not the most profitable technique for the treatment of plastic waste,
it will have a significant role in the future. In spite of the application of clean technologies and waste
elimination, it is not expected that the amounts of plastic wastes will decline, thus, new recycling
methods will have to be developed. From the perspective of catalysis, chemical recycling of plastic
wastes is the most noteworthy of plastic waste recovery techniques.

2
Energy recovery
Waste incineration, or controlled burning, is typically considered as a disposal method, because it is
usually applied as a method of reducing the volume of miscellaneous municipal waste. However,
incineration of plastics can also be seen as recovery method, as plastics could replace the application
of other oil based fuels. It can be viewed that the plastic application is the first purpose of oil, and
energy production is the secondary task. Indeed incineration with energy reclamation is considered as
a recovery method and, due to their high energy content, plastic waste is a valuable fuel. The heat
capacity of plastics and some other materials are shown in the table 1.
Table 1 Heat capacity of plastics and some other materials (Ylä-Mella 2002).
Material Heat
capacity
[MJ/kg]
Material Heat
capacity
[MJ/kg]
PVC
PE
PET
PS
ABS
18
27
46
41
35
Heavy fuel oil
Coal
Natural gas
Milled peat
Paper
41
26
36 *
10
17
* Unit MJ/m
3
(0
°C)
Mechanical recycling
Plastics can also be recovered from waste via mechanical recycling. The mechanical recycling process
involves a number of operational steps: separation of plastics by resin type, washing to remove dirt
and contaminants, grinding and crushing to reduce the plastics’ particle size, extrusion by heat and
reprocessing into new plastic goods. This type of recycling is mainly restricted to thermoplastics
because thermosets cannot be remoulded by the effect of heat. (Aguado and Serrano 1999)
Mechanical recycling of plastics is limited by the compatibility between the different types of
polymers. Presence of a polymer dispersed in a matrix of a second polymer may dramatically change
the properties and hinder the possibilities to use it in the conventional applications. A good example of
this is the impacts of polyvinyl chloride (PVC) during polyethylene terephthalate (PET) processing.
Only a small amount of PVC in the recycled PET strongly reduces the commercial value of the latter.
(Aguado and Serrano 1999) Another problem with mechanical recycling is the presence in plastic
waste of products made of the same resin but with different colour, which usually impart an
undesirable grey colour to the recycled plastic. (Aguado and Serrano 1999)
In addition, most polymers suffer certain degradation during their use due to effects of temperature,
ultraviolet radiation oxygen and ozone. Therefore, recycled polymers exhibit lower properties and
performance than the virgin polymers, and are useful only for undemanding and lesser value
applications. Recycling of plastics without prior separation by resin produces a material with
mechanical properties similar to timber. Hence, it is often used for the replacement of timber in certain
applications. A higher quality of recycled plastics is achieved when separation by resin is carried out
prior to the remoulding step. (Aguado and Serrano 1999) Stages and their relations in the mechanical
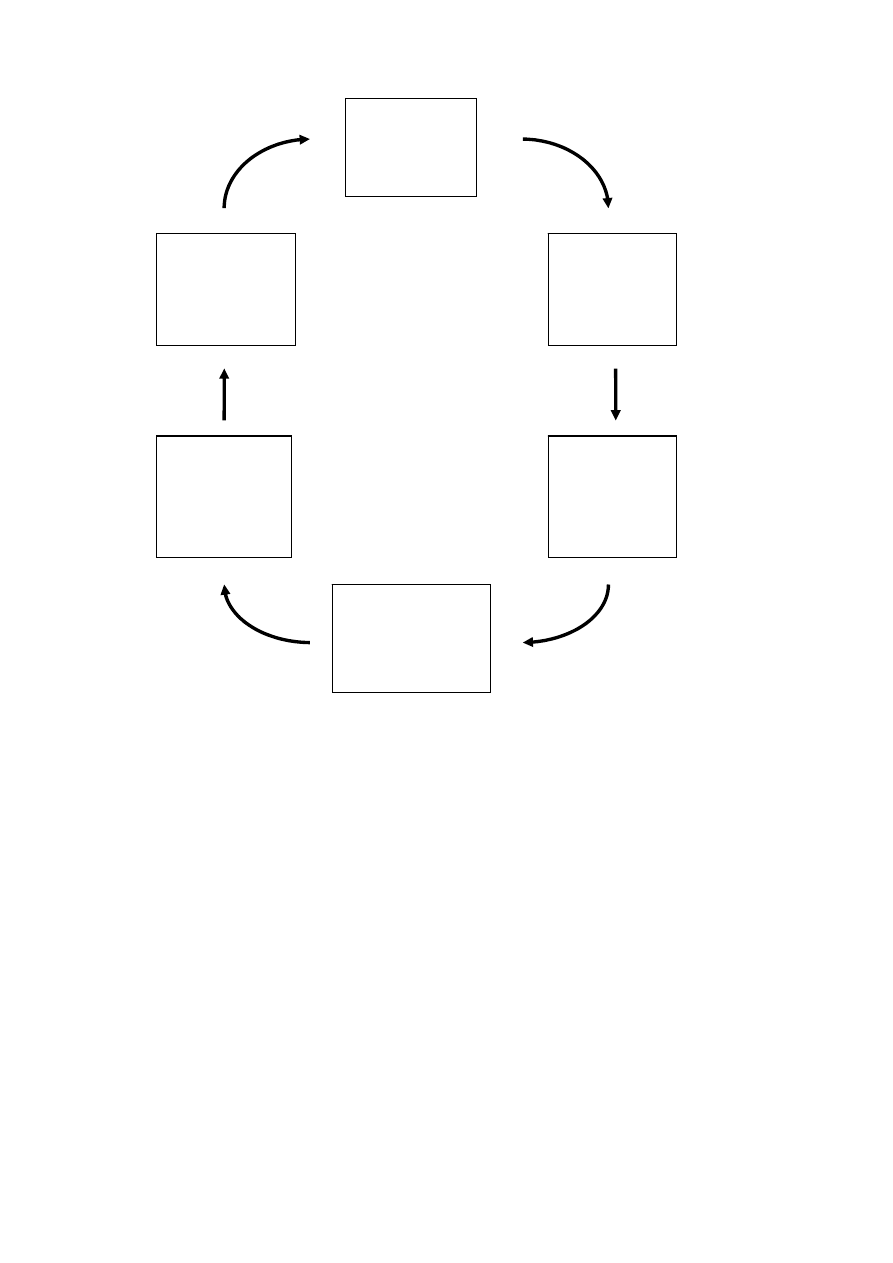
recycling of plastics are shown in Figure 2.
3
B Reclamation
- Grinding
- Washing
- Separating
- Classification
- Quality control
C Homogenizing
- Storage
- Mixing
- Conveying
- Pelletizing
- Quality control
D Compounding
- Blending
- Reinforcing
- Filling
- Modifying
- Stabilizing
- Quality control
E Marketing
- Application development
- Specification
- Design
- Availability
- Quality constancy
- Price
F Use
- Processor
- System
manufacturers
- Sales
- First user
- Maintenance/repair
- Last user
A Logistics
- Transportation
- Collection
- Disassembly
- Sorting
- Shredding
- Quality control
Used
Product
Base recyclable
Shredded
material
Specified
regrind
Recyclable/
Virgin material
Application
Figure 2 Stages in the mechanical recycling of plastics (Burgdorf et al. 1997).
Feedstock recycling
Feedstock recycling of plastics, also referred to as chemical or tertiary recycling is based on the
decomposition of polymers by means of heat, chemical, or catalytic agent, to yield a variety of
products ranging from the chemical monomers to a mixtures of compounds with possible applications
as a source of chemicals or fuels. (Aguado and Serrano 1999) The chemical recycling processes can be
classified into three main areas (Janssen and van Santen 1999):
1. Recycling to fuels (gasoline, liquefied petroleum gas (LPG) and diesel oils)
2. Recycling to monomers
3. Recycling to industrial chemicals.
Depending on recyclable plastic types, desired composition and molecule weight of products, many
different methods of feedstock recycling can be implemented within above areas. (Ylä-Mella 2002,
Janssen and van Santen 1999). For example, Figure 3 illustrates the methods for the feedstock
recycling of plastics and rubber.
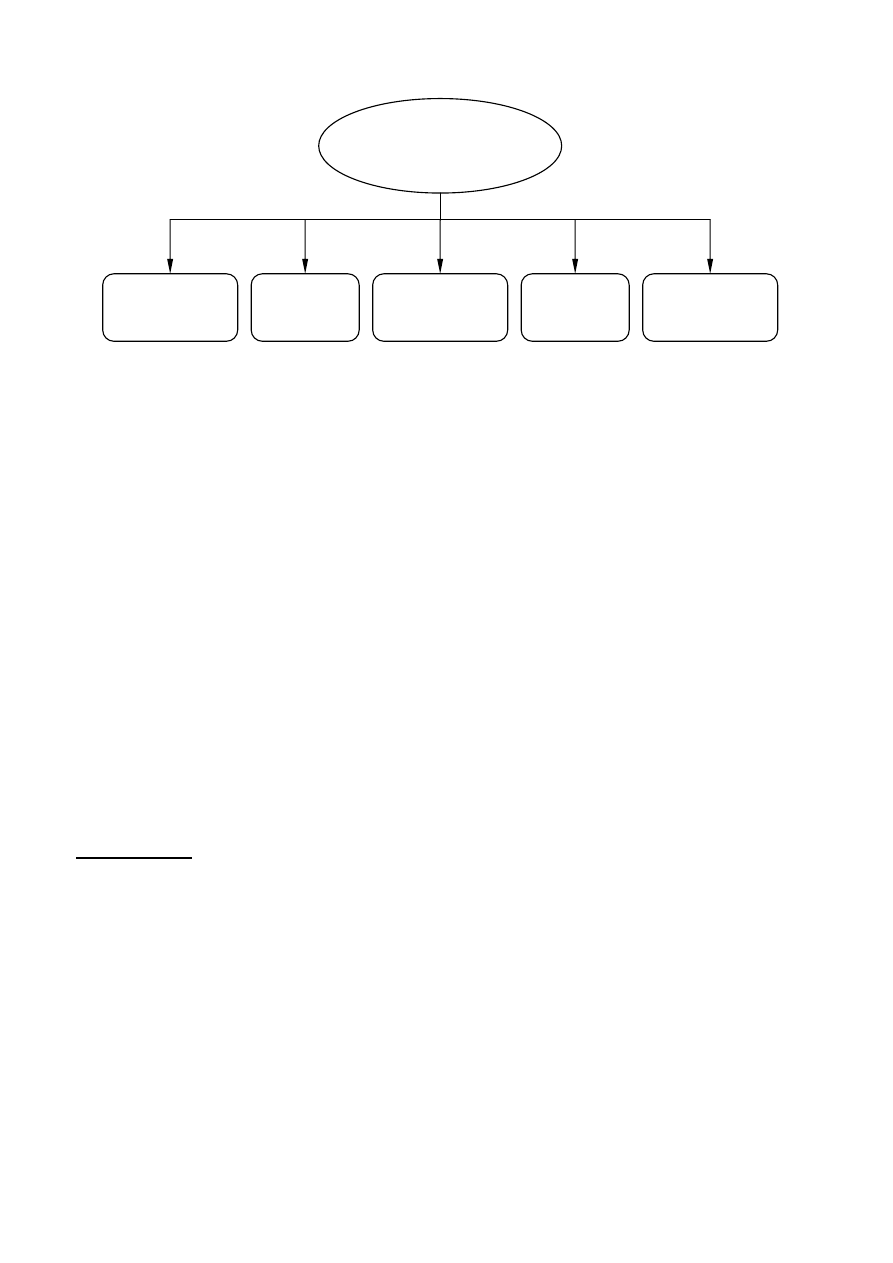
4
Chemical
depolymerization
Gasification
Thermal
treatments
Catalytic cracking
and reforming
Plastics and rubber wastes
Hydrogenation
Figure 3 Alternatives for the feedstock recycling of plastics and rubber wastes (Aguado and Serrano
1999)
Up till now. only a small number of chemical recycling methods have been commercially realized but
the interest in more efficient processes is still growing due to the emerging need of polymer waste
recycling in the future. At the present, feedstock recycling is more limited by process economy than
by technical reasons. The factors which determine the profitability of alternative feedstock recycling
methods are the degree of separation required in raw wastes, the value of the products obtained, and
the capital investments in the processing facilities. (Aguado and Serrano 1999)
According to the separation steps required, the methods can be ordered as follows: gasification <
thermal treatments hydrogenation < catalytic cracking < chemical depolymerization. However, the
feedstock methods can be ordered also according to the commercial value of the products. In that case,
the order of methods will be as follows: thermal oils
≈
≈
synthesis gas < hydrogenation oils
≈
catalytic
oils < monomers. It is interesting to note that the required pre-treatments and product value follow
almost reverse orders. (Aguado and Serrano 1999)
However, comparison of required pre-treatments and product value is not enough. Many other factors
should be included for an adequate comparison of these methods. For instance the possibility of
carrying out the treatment in existing or new facilities, minimum size of the industrial plants needed to
be profitable, required investments and plants location are such factors. (Aguado and Serrano 1999)
Hydrogenation
Hydrogenation of plastics is a potential alternative for breaking down the polymer chain. Compared to
treatments in the absence of hydrogen, hydrogenation leads to the formation of highly saturated
products, avoiding the presence of olefins in the liquid fractions, which favours their use as fuels
without further treatments. Moreover, hydrogenation promotes the removal of hetero atoms, such as
chlorine (Cl), nitrogen (N) and sulphur (S), in the form of volatile compounds. However,
hydrogenation suffers several drawbacks, mainly due to the cost of hydrogen and the need to operate
under high pressure. (Aguado and Serrano 1999)
Even though some non-catalytic hydrogenation processes have been developed, most of the
hydrogenation processes require the presence of bi-functional catalysts to promote hydrogen addition
reactions. A typical catalyst in the hydrogenation includes transition metals, such as palladium (Pt),
nickel (Ni), molybdenum (Mo) and iron (Fe), supported on acid solids such as alumina, zeolites or
amorphous silica-alumina. (Aguado and Serrano 1999)

5
Gasification
Gasification can be considered to be a partial oxidation process of carbonaceous material leading
predominantly to a mixture of carbon monoxide (CO) and hydrogen (H
2
). It is also called synthesis
gas or syngas because of its application in a variety of chemical synthesis. Gasification has been
initially developed for coal conversion, but it has been further applied also to the processing of heavy
petroleum fractions and natural gas. (Aguado and Serrano 1999)
Gasification is an efficient treatment for polymeric waste because of its several advantages: it is not
necessary to separate the different polymer types, and it is possible to mix plastic wastes with other,
non-plastic solid waste before gasification. However, the profitability of a gasification process largely
depends on the value and applications of the synthesis gas. Syngas can be used for the synthesis of
various chemicals, such as methanol, ammonia or acetic acid, but it can also be burned in combustors.
However, incineration of synthesis gas cannot be really considered as a feedstock recycling of plastics,
rather it is considered as a means of energy recovery. (Aguado and Serrano 1999)
When oxygen or air is used as a gasification agent, the content of agent in the reaction must be kept
low, in order to avoid complete oxidation into carbon dioxide and water. Gasification can be promoted
by metal catalyst, which is typically added in aqueous solutions. (Aguado and Serrano 1999) The basic
reactions during gasification of carbonaceous material are shown in scheme 1.
Scheme 1 Basic reactions during the gasification of a carbonaceous material (Aguado and Serrano
1999).
6
The principles of gasification of pure polymeric wastes are similar to the gasification of carbonaceous
material. However, certain details have to be taken into account when plastic and rubber wastes are
processed. For instance, the heterogeneity of the starting materials, the problem of feeding the highly
viscous melted plastics, and the possible formation of corrosive compounds such as hydrochloric acid
(HCl) and polyvinyl chloride (PVC), are some examples of details that have to be taken into
consideration. (Aguado and Serrano 1999)
Chemical depolymerization
During the chemical depolymerization process, the polymer is cracked to the original monomer in the
presence of different reagents. Recycled monomers are identical to those used in the preparation of
virgin polymers, consequently, plastics prepared from both fresh monomers and depolymerization end
products have similar characteristics and quality. (Aguado and Serrano 1999)
Chemical depolymerization is the most established method of plastic feedstock recycling, even though
it is restricted to the recycling of condensation polymers and there are no applications of
decomposition of other polymers. The total volume of condensation polymers accounts for less than
15 % of all plastic wastes. As examples of common condensation polymers, polyesters, polyamides
and polyacetals can be mentioned. Condensation polymers are obtained by the random reaction of two
molecules, which proceeds with the liberation of a small molecule as the chain bonds are formed. In
the chemical depolymerization, the reverse reaction of polymer formation takes place through the
reaction of those small molecules with the polymeric chains. Depending on the chemical agent used to
break down the polymer, different depolymerization routes are envisaged: for instance glycolysis,
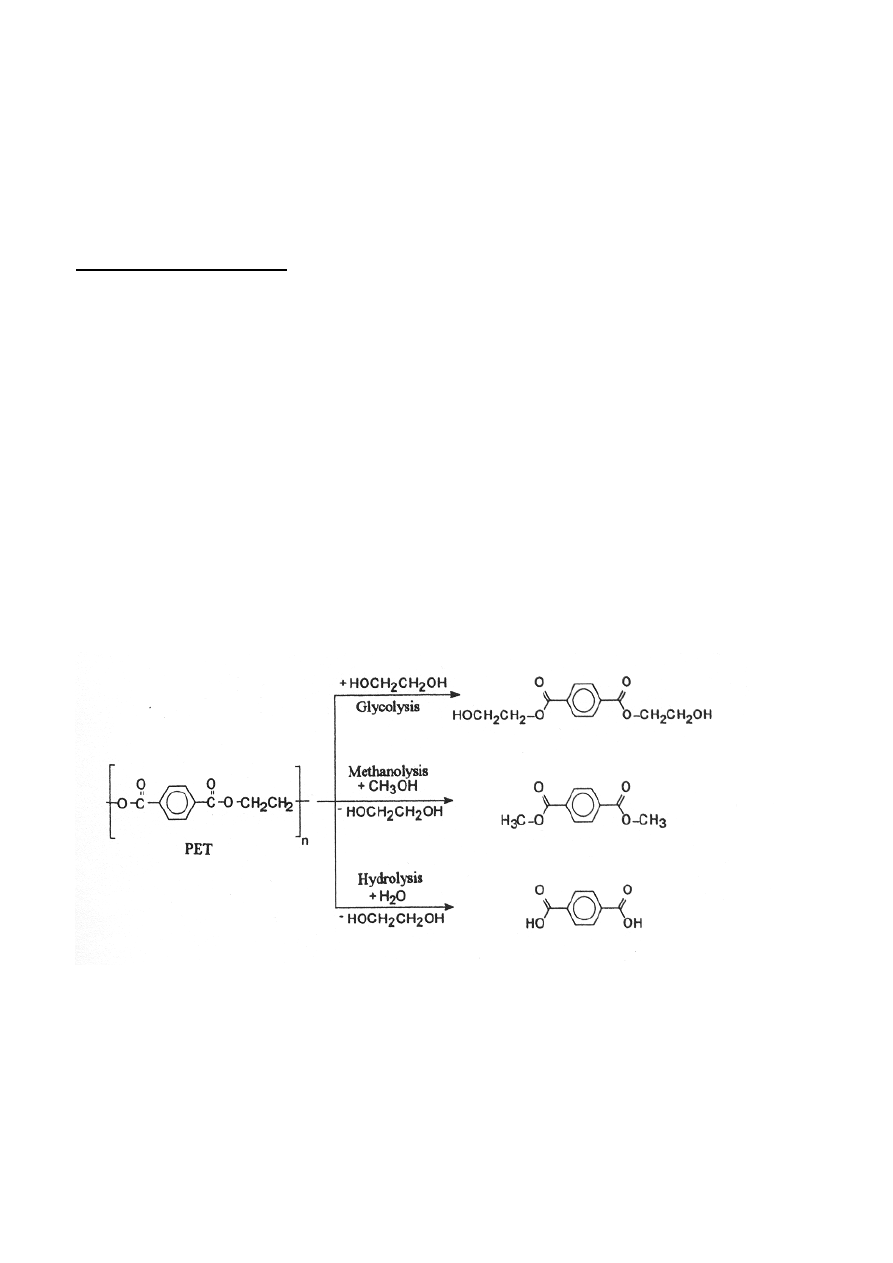
methanolysis, hydrolysis and ammonolysis. (Aguado and Serrano 1999) An example of
depolymerization of polyethyleneteraphtalate (PET) is shown in Figure 4.
Figure 4 Depolymerization of PET into monomers by different solvolysis methods. (Janssen and van
Santen (1999)
Some promising alternatives of chemolysis have been also found through a combination of different
treatments. The Ford hydroglycolysis process is a good example of these combined alternatives, as it
couples hydrolytic and glycolytic reactions to degrade the polyurethane chains. Other combinations of
chemolysis have also been studied. (Aguado and Serrano 1999)

7
Thermal treatment
Thermal treatment is a collective term to describe different methods and processes developed for
breaking down polymeric materials simply by treatment at high temperature in an inert atmosphere.
They are mainly used for the feedstock recycling of addition polymers, whereas condensation
polymers are preferably depolymerised by reaction with certain agents. (Aguado and Serrano 1999)
Thermal decomposition of polymers can be considered as a depolymerization process in only a few
cases, given that thermal decomposition of most polymers leads to a complex mixture of products,
containing low monomer concentrations. The types and distribution of products derived form the
thermal degradation of each polymer depend on a number of factors: the polymer itself, the reaction
conditions and the type and operation mode of the reactor, for instance. (Aguado and Serrano 1999)
There is some confusion regarding a thermal treatment of polymers is to be described as
depolymerization, cracking, thermolysis or pyrolysis. For example, the term pyrolysis refers to the
thermal decomposition of polymeric material at high temperatures (above 600 ºC), whereas thermal
cracking refers to degradation at lower temperatures. However, in some cases, the process is not
confined to any of the above process characteristics, for instance in the case when the temperature is
continuously varied. In this situation it is difficult to assign one term to be used to describe the
process. (Aguado and Serrano 1999)
Thermal degradation of plastics and rubber proceeds through a radical mechanism, which may involve
three different decomposition pathways (Aguado and Serrano 1999):
1. Random scission at any point in the thermal backbone leading to the formation of smaller
polymeric fragments as primary products, which in turn may be subjected to additional
random cracking reactions.
2. End-chain scission, where a small molecule and a long-chain polymeric fragment are
formed. If the small molecule released is the starting monomer, the thermal degradation
process can be considered as an actual depolymerization or unzipping process.
3. Abstraction of functional substituents to form small molecules. In this case, the polymer
chain may retain its length or the release of the small molecule may be accompanied by
cleavage of the polymeric chain.
In many cases, several of these pathways occur simultaneously. During the thermal degradation of
many polymers, other reactions may also occur at the same time. For instance during the cracking
reactions isomerization, cyclization, aromatization and recombination can also take place. Thus, an
increase in the degree of branching of the polymeric chains is usually observed, as they are reduced in
length by thermal decomposition. (Aguado and Serrano 1999)
Catalytic cracking and reforming
Catalytic cracking and reforming of plastic wastes are based on contact of the polymer with a catalyst
that promotes its cleavage. In fact, plastic degradation proceeds in most cases by a combination of
catalytic and thermal effects, which cannot be isolated. Beside catalytic cracking, the use of catalysts
is usual also in other earlier mentioned processes, such as gasification and partial oxidation of plastics.
However, there is no chemical agent incorporated to react directly with the polymer during the
catalytic cracking process and the products derived from the polymer decomposition are not usually
the starting monomers. (Aguado and Serrano 1999)

8
There are many advantages in catalytic cracking compared to thermal cracking. For example, polymer
molecules start to break down at lower temperatures. In consequence of the lower temperature, the
energy requirement is also lower. Further, if the rates of reactions between catalytic and thermal
cracking are compared, the catalytic process is faster than the thermal process because of lower
activation energy. Using of catalysts also improves the quality and selectivity of products because the
product distribution can be varied and controlled by the selected catalysts. (Ylä-Mella 2002)
All these factors illustrate the great potential of catalytic cracking for the conversion of polymeric
wastes into valuable components. However, this method also suffers from drawbacks and problems,
which are still not completely solved. For instance with time, the catalysts are deactivated by the
decomposition of carbonaceous residues, and by poisons present in the raw waste stream such as
chlorine (Cl) and nitrogen (N) compounds. Moreover, the inorganic compounds contained in the
plastic wastes tent to remain with the catalysts, hindering their later recovery and re-use. For these
reasons, catalytic cracking is mainly applied to polyolefinic wastes of relatively high purity, requiring
a number of pre-treatment steps to remove compounds that may negatively affect the catalysts.
(Aguado and Serrano 1999)
Other difficulties arise from the high viscosity of the molten plastic, which hinders its flow through
conventional fixed bed reactors. These problems are largely avoided when the catalytic conversion is
combined with a simple thermal treatment, aimed at reducing the viscosity of the mixture and enabling
the separation of unwanted components. (Aguado and Serrano 1999)
A wide variety of catalysts have been found effective in promoting the decomposition of plastics
materials: Friedel-Crafts catalysts, acidic and basic solids, bi-functional solids, etc. The most common
catalysts used in plastics cracking are acidic solids, mainly alumina, amorphous silica-alumina and
zeolites. These catalysts are typically used in petroleum processing and by petrochemical industries.
They have very different textural and acidic properties, which directly determine their catalytic
activity and product selectivity. This is an important factor, because the initiation step of polymer
catalytic degradation depends on the type of acid sites and leads to different to cracking pathways.
(Aguado and Serrano 1999)
Conclusions
Plastics have become common materials of our everyday lives and many of their properties contribute
to sustainable development. However, at the end of their useful life, plastics waste may cause a waste
management challenge. This problem is aggravated by the fact that plastic applications are often used
only once before disposal.
Waste incineration, or controlled burning, is typically considered as a disposal method because of its
application for a mere reduction of the volume of waste. However, incineration with energy recovery
is considered as a recovery methods, as plastics can replace other oil based fuels.
The polymers in plastics can be recovered via mechanical recycling. This process involves a number
of operations including separation of plastics by resin-type, washing to remove dirt and contaminants,
grinding and crushing to reduce the plastics particle size, extrusion by heat, and reprocessing into new
plastic goods. This type of recovery is mainly restricted to thermoplastics, because thermosets cannot
be remoulded by the application of heat.
The chemical recycling processes can be classified into recycling to fuels, monomers or industrial
chemicals. During chemical recycling processes, plastic wastes can be remanufactured into valuable
chemical feedstock by a large variety of thermal or catalytic processes. Thermal processes are less
9
sensitive than catalytic processes to dirt and critical impurities, such as Cl, S, N and heavy metals,
however, the end products are mostly of lower quality and of lesser value. In the future, catalysis may
offer an important contributions to the efficiency of feedstock recycling, provided that the problems of
catalyst deactivation by contaminants can be overcome in an economic viable way.
The alternative methods for feedstock recycling of plastic and rubber wastes can be summed up into
the following classes:
1. Hydrogenation
The polymer is degraded by the combined actions of heat, hydrogen and many cases catalysts.
2. Gasification
Plastic wastes react with oxygen and/or steam to produce synthesis gas (CO and H
2
).
3. Chemical depolymerization
Plastic wastes react with certain agents to yield the starting monomers.
4. Thermal cracking
Plastic wastes are decomposed by the effect of heat in an inert atmosphere.
5. Catalytic cracking and reforming
The polymer chains are broken down by the effect of catalyst, which promotes cleavage
reactions.
References:
Aguado J & Serrano D (1999) Feedstock Recycling of Plastic Wastes. Royal Society of Chemistry,
Clean Technology Monographs. Cambridge, UK.
Burgdorf P, Keller B & Orth P (1997) Computer housings in material recycling loop.
< http://www.plastics.bayer.com/bayer/ > (28.9.2001)
Janssen FJJG & van Santen RA (1999) Environmental Catalysis. Imperial College Press, Netherlands
Institute for Catalysis Research. London, UK.
Phillips PS (2000) Practical techniques for waste management. University of Oulu. Industrial Ecology
course for the Graduate School in Chemical Engineering. 22
nd
- 26
th
May 2000.
Strong AB (2000) Plastics Materials and Processing. Prentice-Hall, New Jersey, USA.
Ylä-Mella J (2002) Recycling of Plastics from the Waste Electrical and Electronic Equipment
(WEEE). University of Oulu, Department of Process and Environmental Engineering, Finland.
Document Outline
- RECYCLING OF POLYMERS
- Energy recovery
- Mechanical recycling
- Feedstock recycling
- Conclusions
- References:
Wyszukiwarka
Podobne podstrony:
plastiki sprawko id 362078 Nieznany
plastiki sprawko id 362078 Nieznany
Abolicja podatkowa id 50334 Nieznany (2)
4 LIDER MENEDZER id 37733 Nieznany (2)
katechezy MB id 233498 Nieznany
metro sciaga id 296943 Nieznany
perf id 354744 Nieznany
interbase id 92028 Nieznany
Mbaku id 289860 Nieznany
Probiotyki antybiotyki id 66316 Nieznany
miedziowanie cz 2 id 113259 Nieznany
LTC1729 id 273494 Nieznany
D11B7AOver0400 id 130434 Nieznany
analiza ryzyka bio id 61320 Nieznany
pedagogika ogolna id 353595 Nieznany
Misc3 id 302777 Nieznany
cw med 5 id 122239 Nieznany
więcej podobnych podstron